By Vince Giuliano and Steve Buss
Image source – retinal neuron
This is the fifth blog entry on a natural process of aging in reverse we have called YOUNGING 1.0. It is about a category of practical interventions that humans can use to initiate YOUNGING 1.0 processes in themselves – interventions based on oxygen scarcity in cells, known as hypoxia. The blog entry also identifies a hitherto undiscussed underlying mechanism explaining hypoxic hormesis – a process that involves remarkable health benefits flowing from various temporary conditions of hypoxia in the body, such as wearing a pressure cuff that limits circulation in an arm for a short period of time.
We suggest that readers new to the YOUNGING 1.0 concept review some of the earlier entries for context before seeking to digest this one. We suggest you start by reading the less technical introductory blog entry YGA Introduction to the YOUNGING Series – Emerging Aging Reversal Strategies and Treatments. After that, the next thing we suggest you review is the substantive blog YGB YOUNGING1.0 – THE EMERGING AGING REVERSAL STRATEGY. Then you can look at the blog entry YGC MORE ON YOUNGING1.0 – THE EMERGING AGING REVERSAL STRATEGY. And finally, the previous entry YGD YOUNGING1.0 PART 4: UNDERLYING MECHANISMS OF YOUNGING 1.0. HSC STEM CELL DIFFERENTIATION.
SUMMARY
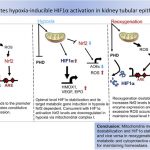
These previous blog entries have been mainly focused on the underlying biological mechanisms through which YOUNGING 1.0 processes produce a variety of phenomena that collectively can be described as a form of aging in reverse. However, these blog entries have not focused on how YOUNGING 1.0 can be reliably and safely initiated in mammals such as ourselves. This particular blog entry is focused on a class of interventions which result in body cells experiencing a lack of oxygen and responding as they are evolutionarily programmed to do, by upgrading a certain cellular signaling pathway known as Hypoxia Induced Factor 1alpha (HIF-1 α).
Since oxygen is so essential for survival of animal cells and bodies, evolution has provided us with a number of mechanisms to protect our cells against real or perceived oxygen insufficiency. The HIF-1α pathway is primary among these. Given a bodily perception of drop in the environmental oxygen level or oxygen insufficiency, the pathway leads to the rapid activation of numerous protective and health-promoting genes – a situation known as hypoxic hormesis. Studies have revealed many possible benefits including hippocampal neurogenesis, strengthend immune response, lessened inflammation, decreased arterial hypertension, improved wound healing, better capacity to exercise and enhanced spatial learning and memory)(ref).
“Eventually, HIF-1 acts as a master regulator of numerous hypoxia-inducible genes under hypoxic conditions. The target genes of HIF-1 encode proteins that increase O2 delivery and mediate adaptive responses to O2 deprivation. Despite its name, HIF-1 is induced not only in response to reduced oxygen availability but also by other stimulants, such as nitric oxide and hydrogen sulfide, and various growth factors.(ref HIF-1 Signaling Pathway)
Among the known impacts of activation of that pathway is genome-wide demethylation of the H3K27me2-3 histone via expression of JMJD3. And that, in turn has been argued in previous blog entries to be the central activating mechanism of YOUNGING 1.0. JMJD3 activation can results in the re-activation of literally thousands of genes, including many involved in growth and development as well as DNA repair and other kinds of cellular damage remediation. Finally, in laying this all out, it becomes evident that the YOUNGING process itself may be responsible for the health benefits longer known to result from intermittent hypoxia (hypoxic hormesis)(ref).
Temporary Induction of hypoxia is increasingly recognized in the research literature as being at the nexus of epigenetic regulation for numerous biological aging as well as growth and development and regenerative phenomena, including inflammation, hematopoiesis, wound healing, angiogenesis, inflammation, aging, and cell senescence. We believe that is because hypoxia induces expression of JMJD3, which leads to H3K27me2-3 histone demethyation which turns on thousands of genes as part of a YOUNGING 1.0 process.
ON THE EVOLUTIONARY DEVELOPMENT OF BIOLOGICAL MECHANISMS TO PROTECT OXYGEN AVAILABILITY
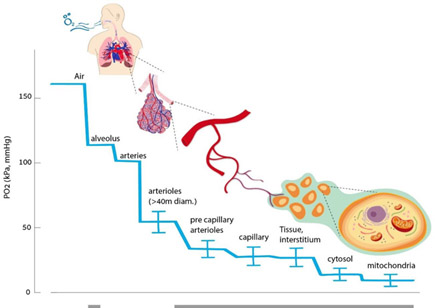
From the 2020 publication The Hyperoxic-Hypoxic Paradox “Oxygen is the third-most abundant element in the universe, after hydrogen and helium, and it is the most dominant effector of most living creatures on earth. About 300 million years ago, during the Carboniferous period, atmospheric oxygen levels reached a maximum of 35%, which may have contributed to the large size of animals and insects at this time [1,2]. Today, oxygen constitutes 20.8% of the earth’s atmosphere, and any slight change in its concertation will have a dramatic impact on all levels of mammalian physiology. The ability to maintain oxygen homeostasis is essential for survival, and all mammalian physiological systems evolved to ensure the optimal level of oxygen supplied to all cells in each organism. This has transpired through the evolution of a complex physiological infrastructure for oxygen delivery (the lungs), oxygen transport carriers (erythrocytes and plasma), oxygen transport pathways (vascular system), and the pump (heart). Both the development and regulation of these systems in organisms provide the basis for oxygen homeostasis — Accordingly, low levels of oxygen, or hypoxia, are one of the most powerful inducers of gene expression, metabolic changes, and regenerative processes, including angiogenesis and stimulation of stem-cell proliferation, migration, and differentiation.” The mechanisms of the hypoxic response include basic ones of gene regulation moderated, as we shall see, by histone methylation as a way of simultaneously affecting large numbers of genes. This diagram from the same publication illustrates the oxygen delivery chain.
JMJD3 IS ACTIVATED BY HYPOXIA
A central message of this blog entry is that hypoxia leads to expression of JMJD3 and consequent demethylation of H3K27me3,. We therefore strongly suspect that hypoxia is an important initiator of YOUNGING 1.O. The 2014 publication HIF-1-Dependent Induction of Jumonji Domain-Containing Protein (JMJD) 3 under Hypoxic Conditions reports: “Jumonji domain-containing proteins (JMJD) catalyze the oxidative demethylation of a methylated lysine residue of histones by using O2, α-ketoglutarate, vitamin C, and Fe(II). Several JMJDs are induced by hypoxic stress to compensate their presumed reduction in catalytic activity under hypoxia. In this study, we showed that an H3K27me3 specific histone demethylase, JMJD3 was induced by hypoxia-inducible factor (HIF)-1α/β under hypoxia and that treatment with Clioquinol, a HIF-1α activator, increased JMJD3 expression even under normoxia. Chromatin immunoprecipitation (ChIP) analyses showed that both HIF-1α and its dimerization partner HIF-1β/Arnt occupied the first intron region of the mouse JMJD3 gene, whereas the HIF-1α/β heterodimer bound to the upstream region of the human JMJD3, indicating that human and mouse JMJD3 have hypoxia-responsive regulatory regions in different locations. This study shows that both mouse and human JMJD3 are induced by HIF-1.” —
“In response to hypoxia, cells express several genes to maintain homeostasis. More than 500 genes, including vascular endothelial growth factor (VEGF), erythropoietin (EPO), phosphoglycerate kinase-1 (PGK-1), glucose transporter-1 (glut-1), and BCL-2/adenovirus E1B 19 kDa interacting protein 3 (Bnip3) are induced under hypoxia in MCF7 cells (Elvidge et al., 2006). These diverse target genes are transcribed by a common transactivator, the hypoxia-inducible factor 1-α/β (HIF-1α/β) heterodimer (Semenza, 2012). Under normoxic conditions, HIF-1α is ubiquitinated and rapidly degraded whereas HIF-1β is constitutively expressed. HIF-1β is identical to the Aryl hydrocarbon receptor nuclear translocator (Arnt) that is a dimerization partner protein of the dioxin receptor, Aryl hydrocarbon receptor. The 403rdand 564th proline residues of human HIF-1α are hydroxylated by prolyl hydroxylase domain protein (PHD) (Bruick and McKnight, 2001). The hydroxylated prolines are recognized by the E3 ubiquitin ligase von Hippel-Lindau protein (pVHL), which triggers the ubiquitination-dependent degradation of HIF-1α (Maxwell et al., 1999). The 803rd asparagine residue of HIF-1α is also hydroxylated by an oxygen-dependent asparagine hydroxylase, referred to as Factor-Inhibiting HIF-1 (FIH-1). The hydroxylated asparagine residue hinders the recruitment of the CREB-binding protein (CBP)/p300 coactivator. PHD and FIH-1 require molecular oxygen, α-ketoglutarate, vitamin C, and Fe(II) for their catalytic activities. The trans-active form of HIF-1α is stabilized under hypoxia because the activities of these two oxygen-dependent hydroxylases decrease (Hewitson et al., 2002).” —
“More than 100 JMJDs have been identified, and most of them have demethylase activity with different substrate specificities (Shi and Whetstine, 2007). JMJD1A/JHDM2A/KDM3A demethylate H3K9me2 and me1, JMJD2 isozymes demethylate H3K9me3, Jarid1 isozymes demethylate H3K4me3, and JMJD3/KDM6B demethylate H3K27me3. Histone methylation affects gene expression in distinct localized patterns. The tri-methylation of H3K4 and acetylation of H3 in the promoter region are associated with actively transcribed genes, whereas the methylation of H3K9 and H3K27 in the promoter region are associated with inactive genes (Kooistra and Helin, 2012). The fact that JmjC proteins need molecular oxygen for their catalytic activities suggests that their activities can be inhibited under hypoxic conditions. Recent findings that hypoxia induced several JmjC proteins imply that their induction compensates their reduced catalytic activity under hypoxia (Beyer et al., 2008; Pollard et al., 2008; Wellmann et al., 2008; Xia et al., 2009). Thus, in response to hypoxic stress, histone methylation of individual genes can be dynamically regulated by the net change in the activity and expression of the JmjC proteins.” —
JMJD2s, JMJD1A, and Jarid1B have been identified as HIF-1α target genes (Beyer et al., 2008; Pollard et al., 2008; Xia et al., 2009). This study first demonstrated that hypoxia also induces an H3K27me3 demethylase, JMJD3, by a HIF-1α dependent mechanism. Together with the recent findings that JMJD3 is involved in in breathing, inflammation, cardiac development, neural development, and senescence, this study suggests that hypoxia can influence these diverse biological processes by changing the expression level of JMJD3”
IMPACT OF JMJD3 ON GENE ACTIVATION
As discussed in previous blog entries of this series. JMJD3 demethylates H3K27me2-3 histones activating thousands of silenced genes. The actions of jmjd3 are illustrated in this diagram from the publication JMJD3 in the regulation of human diseases:
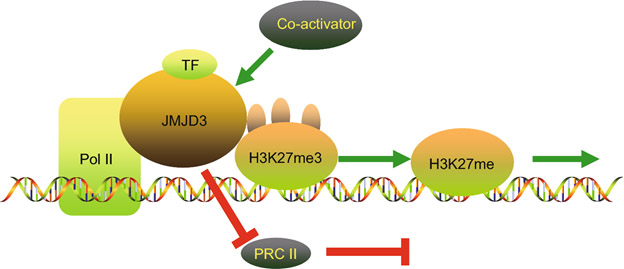
“A graphical illustration on the mechanisms of JMJD3 in the regulation of enhancing gene transcription. JMJD3 promotes gene transcription in a demethylase-dependent or independent manner. JMJD3 enhances the initiation of transcription through three ways. Firstly, JMJD3 demethylate H3K27me3 on the promoter of targeted genes. Secondly, JMJD3 releases suppressive PRC2 complexes. Thirdly, JMJD3 binds to the Pol II elongation complex, enhancing its progression through H3K27me3-enriched gene bodies to enhance transcription elongation. This is the demethylase-dependent process. JMJD3 recruits co-activators to targeted gene promoters by interacting with both the co-activator and transcription factors in gene promoters. This co-activation process does not require demethylase activity. This is the demethylase-independent manner.”
CONTEXT OF HYPOXIC JDJM3 ACTIVATION
The impacts of hypoxia and JDJM3 activation can be either detrimental as in the case for several disease conditions and/or quite positive, dependent on site and locale, timing, duration, periodicity and intensity. A small scrape on the skin or insect bite may initiate a strictly local activation associated with local inflammation, as part of a normal wound healing response. Hypoxic Stress on a particular organ or body system such, for example, as induced by strenuous leg exercise may induce a response only or mainly in the legs involved. General hypoxic stress associated with insufficient oxygen while breathing may be communicated by the circulatory system to a multiplicity of body systems and locales leading to a whole body response. The hypox situation in blood cells is carried everywhere the circulatory system goes. Likewise, JMJD3 activation paths associated with the blood circulatory system such as might be encountered in the case of heterochronic parabiosis or umbilical cord plasma infusions will likely produce whole body responses.
The YOUNGING 01 response I we originally characterized in the first blog entry of this series was such a whole-body response. YOUNGING 01 is envisaged as being triggered by whole-body stress lasting from a few minutes to a maximum of a few hours that activates JMJD3, This could be a general hypoxic stress due to, for example. a transition from breathing concentrated oxygen to breathing normal air while exercising. We view this YOUNGING01 activation phase to be transient although it could be repeated periodically. Animal experiments suggest that the YOUNGING 01 process itself is a consequence of the whole body activation of thousands of genes by JMJD3, leading to forms of reverse aging that could take years to play out.
BENEFICIAL INDUCTION OF INTERMITTENT HYPOXIA
It has been known for many years that many actions which temporarily limit oxygen supply to cells and activate hypoxia induced factors can induce a wide variety of health benefits in mammals such as ourselves. This is because hypoxia activates a very large number of genes involved in growth and development including ones connected with angiogenesis, erythropoiesis, cell differentiation and proliferation as illustrated in this diagram. The genes listed are representative; many more are actually involved:
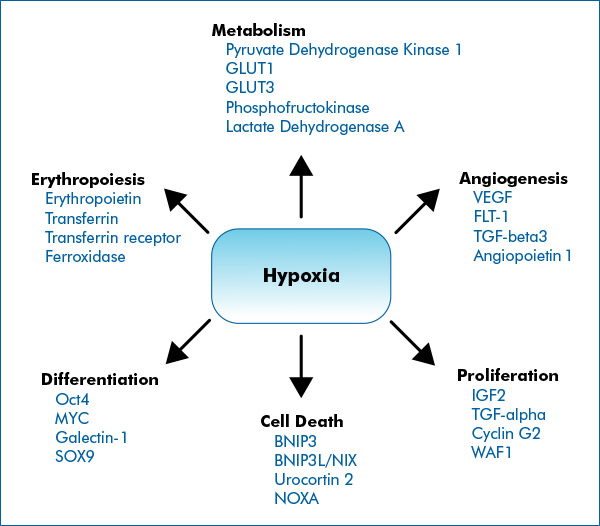
Image and following text source Hypoxia Signaling by Novus Biologicals “ The cellular response to hypoxia is predominantly shaped by HIF signaling. HIFs, primarily HIF-1, HIF-2 and to a less-defined extent HIF-3, control the expression of hundreds of genes involved in key cellular processes that facilitate adaptation to low-oxygenation. — HIFs are considered “Master Regulators of Hypoxia”, because they control the expression of hundreds of genes which help cells to survive under low oxygenation. However, several alternative pathways have been identified to contribute to the overall hypoxia response and cellular adaptations. Often these signaling pathways intersect HIF signaling.” —
HYPOXIA HIF1-INDUCING INTERVENTIONS
Here are some of the most important possible hypoxia HIF1-inducung interventions:
- aerobic exercise
- measures which temporarily restrict blood flow within limbs, such as pressure cuffs and bands
- high altitudes such as above 9000 feet
- stays in decompression chambers
- body accommodation to hyper oxygenation which is suddenly discontinued
- inhibitors of steps in the mitochondrial respiratory chain such as by certain drugs
- vascular constriction induced by drugs or other effects
- a limb “going to sleep” accompanied by loss of sensation and muscular control due to circulatory restriction
- holding one’s breath
Intermittent hypoxia can also result from multiple disease and environmental situations, such as
- obstructive sleep apnoea (OSA)
- diseases such as COPD
- lack of hemoglobin, anemia
- insufficient microvascular dilation due to drugs or insufficient expression of nitric oxide
- environments without sufficient ventilation
- presence of carbon monoxide or cyanide which inhibit normal oxygen conveying functions of hemoglobin
- Serious infection with COVID can impact breathing and can create a life-threatening hypoxic situation
Conversely, actions to ensure an adequate oxygen supply (which tend to preclude expression of HIF-1α) include
- breathing supplemental oxygen
- being enclosed in a hyperbaric chamber
- being on a ventilator machine
- taking Viagra, Cialis or Levitra to induce expression of nitric oxide which promotes micro vascular dilation
Known Health Benefits of Intermittent Hypoxia
There is an extensive body of literature associated with both positive and negative health effects of intermittent hypoxia. The detrimental effects can be associated with multiple disease processes. An example is the case of SARS-COVID as illustrated by this diagram:
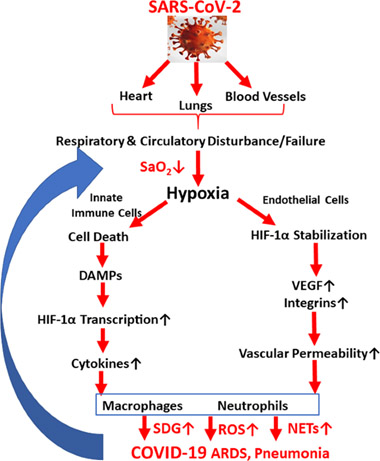
Image source
In this blog entry we are mainly be concerned with positive impacts of temporarily induced by intermittent hypoxia.
THERE IS A GROWING AWARENESS OF THE RELEVANCY OFTHE BENEFITS OF INTERRMITTANT HIF-1α EXPRESSION FOR PROCESSES OF REGENERTIVE MEDICINE
Esentially every process of organ regeneration studied in the field of regenerative medicine requires epigenetic modifications of hundreds of genes from normal steady-as-you-go state into growth-and-development state. Evolution provides central mechanisms for bringing this about. Central to those mechanisms is global H3K27me2-3 demethylation through the expression of JDJM3. And one of the practical ways of inducing this expression is via inducing temporary hypoxia. Therefore it is not surprising that temporary hypoxia is increasingly being recognized as an important tool in the domain of regenerative medicine. The 2021 publication Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling reports. “Historically, the field of regenerative medicine has aimed to heal damaged tissue through the use of biomaterials scaffolds or delivery of foreign progenitor cells. Despite 30 years of research, however, translation and commercialization of these techniques has been limited. To enable mammalian regeneration, a more practical approach may instead be to develop therapies that evoke endogenous processes reminiscent of those seen in innate regenerators. Recently, investigations into tadpole tail regrowth, zebrafish limb restoration, and the super- healing Murphy Roths Large (MRL) mouse strain, have identified ancient oxygen-sensing pathways as a possible target to achieve this goal. Specifically, upregulation of the transcription factor, hypoxia-inducible factor one alpha (HIF-1α) has been shown to modulate cell metabolism and plasticity, as well as inflammation and tissue remodeling, possibly priming injuries for regeneration. Since HIF-1α signaling is conserved across species, environmental or pharmacological manipulation of oxygen-dependent pathways may elicit a regenerative response in non-healing mammals. In this review, we will explore the emerging role of HIF-1α in mammalian healing and regeneration, as well as attempts to modulate protein stability through hyperbaric oxygen treatment, intermittent hypoxia therapy, and pharmacological targeting. We believe that these therapies could breathe new life into the field of regenerative medicine.”Historically, the field of regenerative medicine has aimed to heal damaged tissue through the use of biomaterials scaffolds or delivery of foreign progenitor cells. Despite 30 years of research, however, translation and commercialization of these techniques has been limited. To enable mammalian regeneration, a more practical approach may instead be to develop therapies that evoke endogenous processes reminiscent of those seen in innate regenerators. Recently, investigations into tadpole tail regrowth, zebrafish limb restoration, and the super-healing Murphy Roths Large (MRL) mouse strain, have identified ancient oxygen-sensing pathways as a possible target to achieve this goal. Specifically, upregulation of the transcription factor, hypoxia-inducible factor one alpha (HIF-1α) has been shown to modulate cell metabolism and plasticity, as well as inflammation and tissue remodeling, possibly priming injuries for regeneration. Since HIF-1α signaling is conserved across species, environmental or pharmacological manipulation of oxygen-dependent pathways may elicit a regenerative response in non-healing mammals. In this review, we will explore the emerging role of HIF-1α in mammalian healing and regeneration, as well as attempts to modulate protein stability through hyperbaric oxygen treatment, intermittent hypoxia therapy, and pharmacological targeting. We believe that these therapies could breathe new life into the field of regenerative medicine.”
What we have to add to this and similar discussions are two important new pieces: 1. HIF-1α does its global gene activation job by H3K27me3 demethylation via JMJD3, and 2. Organ regeneration involves a profound aging-reversal process we call YOUNGING 01.
HIF-1 AND ITS BENEFITS (OR PROBLEMS) CAN BE ACTIVATED NOT ONLY BY ACTUAL HYPOXIA BUT ALSO BY ABRUPT CHANGES IN OXYGEN AVAILABILITY.
Again from the 2020 publication The Hyperoxic-Hypoxic Paradox: “Effective metabolism is highly dependent on a narrow therapeutic range of oxygen. — The sensing of decreased oxygen levels (hypoxia) or increased oxygen levels (hyperoxia), occurs through specialized chemoreceptor cells and metabolic changes at the cellular level, which regulate the response. Interestingly, fluctuations in the free oxygen concentration rather than the absolute level of oxygen can be interpreted at the cellular level as a lack of oxygen. Thus, repeated intermittent hyperoxia can induce many of the mediators and cellular mechanisms that are usually induced during hypoxia. This is called the hyperoxic-hypoxic paradox (HHP).”
The following diagram from the publication The Hyperoxic-Hypoxic Paradox illustrates how HIF-1 can be triggered by transitions from normoxia to hypoxia or from hyperoxia to normoxia. Intermittent hyperoxia can trigger HIF-1 multiple times.
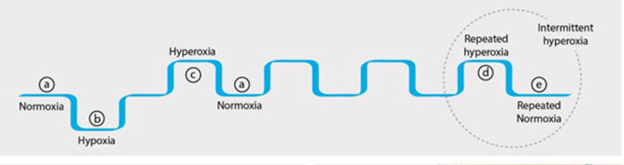
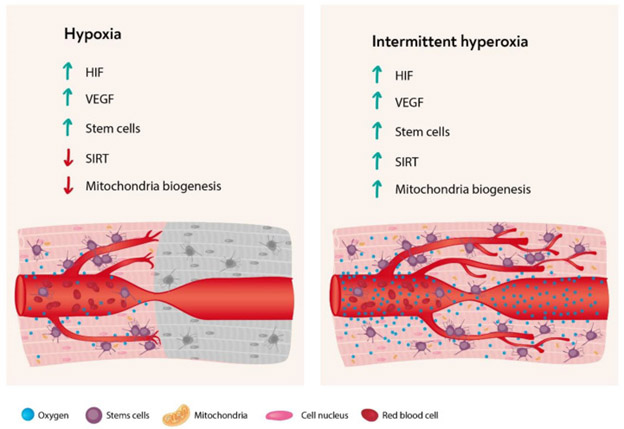
“As summarized in the f0llowing figure (from the same publication) most of the cellular cascades initiated by hypoxia can be induced by intermittent hyperoxia, the so-called “hyperoxic-hypoxic paradox”. HIF, VEGF, SIRT, mitochondrial biogenesis, and stem cell proliferation and migration could all be induced by “biological fooling” the cells with certain protocols of repeated intermittent hyperoxia. Even though the exact dose response-curve has yet to be discovered in clinical practice, certain HBOT protocols have already demonstrated induction of damaged tissue regeneration.”
The same theme is supported by other studies, such as reported in the 2012 publication Pulsed high oxygen induces a hypoxic-like response in human umbilical endothelial cells and in humans: “It has been proposed that relative changes of oxygen availability, rather than steady-state hypoxic or hyperoxic conditions, play an important role in hypoxia-inducible factor (HIF) transcriptional effects. According to this hypothesis describing the “normobaric oxygen paradox”, normoxia following a hyperoxic event is sensed by tissues as an oxygen shortage, upregulating HIF-1 activity. With the aim of confirming, at cellular and at functional level, that normoxia following a hyperoxic event is “interpreted” as a hypoxic event, we report a combination of experiments addressing the effects of an intermittent increase of oxygen concentration on HIF-1 levels and the activity level of specific oxygen-modulated proteins in cultured human umbilical vein endothelial cells and the effects of hemoglobin levels after intermittent breathing of normobaric high (100%) and low (15%) oxygen in vivo in humans. Our experiments confirm that, during recovery after hyperoxia, an increase of HIF expression occurs in human umbilical vein endothelial cells, associated with an increase of matrix metalloproteinases activity. These data suggest that endothelial cells “interpret” the return to normoxia after hyperoxia as a hypoxic stimulus. At functional level, our data show that breathing both 15 and 100% oxygen 30 min every other day for a period of 10 days induces an increase of hemoglobin levels in humans. This effect was enhanced after the cessation of the oxygen breathing. These results indicate that a sudden decrease in tissue oxygen tension after hyperoxia may act as a trigger for erythropoietin synthesis, thus corroborating the hypothesis that “relative” hypoxia is a potent stimulator of HIF-mediated gene expressions.”
Careful attention must be paid to timing, frequency and intensity of doses. “An understanding of proper dosage is needed in order to design an effective intermittent hypoxia protocol, particularly due to the comorbidities associated with hypoxia. For example, intermittent hypoxia has been shown to induce LTF in rats while continuous hypoxia does not.[15] And acute IH shows no evidence of the hippocampal cell death found in rats while chronic intermittent hypoxia exposure does[16] Though intermittent hypoxia has been used for various therapeutic applications across a number of physiological system, there is a general consensus in what can be considered a safe and beneficial amount of intermittent hypoxia. Such a protocol would involve a fraction of inspired oxygen (FiO2) ranging between 0.09 – 0.16 with 3 – 15 episodes per day with comorbidities found in the range of a FiO2 of 0.03 – 0.08 and 48 – 2400 episodes per day.[2] (From Wikipedia)”
THE MASTER INITIATOR OF INFLAMMATION NF-kB IS ACTIVATED BY HYPOXIA
Again ,from Hypoxia Signaling by Novus Biologicals: “NF-kB is a main regulator of inflammatory signaling, orchestrating the induction of proinflammatory proteins including adhesion molecules, cytokines and chemokines. NF-kB signaling is activated via three mechanisms including canonical (dependent on inhibitor of kB kinase -IKK beta), non-canonical pathways (dependent on IKK-alpha) and atypical (IKK-dependent or -independent) pathways. Hypoxia activates NF-kB via the canonical pathway leading to the induction or repression of various target genes.” This table is also from that publication:
While the initialtion of inflammation by hypoxia may seem contra-intuitive, actually it is not surprising since inflammation is the first step in the restorative process of wound healing. Again, I remind you my personal battle against inflammation is not at all against initiation of inflammation; it is against persistent chronic locked-in inflammation typically present in advanced aging.
PRO-INFLAMMATORY CONSEQUENCES OF HYPOXIA ARE DUE IN SOME MEASURE TO JMJD3 ACTIVATION BY HYPOXIA
We know this because knock-down of JMJD3 reduces expression of NF-kB and inflammatory markers. See the articles on this list.
JMJD3 AND NF-kB COOPERATE FOR WOUND HEALING
The 2015 publication Histone H3K27 Demethylase JMJD3 in Cooperation with NF-κB Regulates Keratinocyte Wound Healing reports “Histone H3K27me3 demethylase JMJD3 has been shown to be involved in keratinocyte differentiation and wound healing. However, the exact molecular mechanism underlying JMJD3-mediated keratinocyte wound healing has not been fully elucidated. In this study, we report on the biological function of JMJD3 in keratinocyte wound healing using in vitro cell and in vivo animal models. Our results indicate that JMJD3 up-regulation and NF-κB activation occur in the region of the wound edge during keratinocyte wound healing. We also found that JMJD3 interacts with NF-κB, resulting in increased expression of the inflammatory, matrix metalloproteinase, and growth factor genes via demethylation of H3K27me3 at the gene promoters. Consistently, inactivation of JMJD3 or NF-κB resulted in aberrant keratinocyte wound healing. Our study suggests that regulation of JMJD3 may provide a new therapeutic intervention for treating the chronic skin wound.”
HYPOXIA AND CONSEQUENT EXPRESSION OF JDJM3 IS A MAJOR DRIVER OF ANGIOGENESIS
One of the body’s key regenerative processes is angiogenesis, the development of new vasculature. JMJD3 (KDKM4B) is actively involved and activated by hypoxia, The 2020 publication Hypoxia-Mediated Regulation of Histone Demethylases Affects Angiogenesis-Associated Functions in Endothelial Cells reports: “Objective: Previous studies have demonstrated that the expression of several lysine (K)-specific demethylases (KDMs) is induced by hypoxia. Here, we sought to investigate the exact mechanisms underlying this regulation and its functional implications for endothelial cell function, such as angiogenesis. Approach and Results: We analyzed the expression changes of KDMs under hypoxia and modulation of HIF (hypoxia-inducible factor) expression using GRO-Seq and RNA-Seq in endothelial cells. We provide evidence that the majority of the KDMs are induced at the level of nascent transcription mediated by the action of HIF-1α and HIF-2α. Importantly, we show that transcriptional changes at the level of initiation represent the major mechanism of gene activation. To delineate the epigenetic effects of hypoxia and HIF activation in normoxia, we analyzed the genome-wide changes of H3K27me3 using chromosome immunoprecipitation-Seq. We discovered a redistribution of H3K27me3 at ≈2000 to 3000 transcriptionally active loci nearby genes implicated in angiogenesis. Among these, we demonstrate that vascular endothelial growth factor A (VEGFA) expression is partly induced by KDM4B- and KDM6B–mediated demethylation of nearby regions. Knockdown of KDM4B and KDM6B decreased cell proliferation, tube formation, and endothelial sprouting while affecting hundreds of genes associated with angiogenesis. These findings provide novel insights into the regulation of KDMs by hypoxia and the epigenetic regulation of VEGFA-mediated angiogenesis. Conclusions: Our study describes an additional level of epigenetic regulation where hypoxia induces redistribution of H3K27me3 around genes implicated in proliferation and angiogenesis. More specifically, we demonstrate that KDM4B and KDM6B play a key role in modulating the expression of the major angiogenic driver VEGFA.”
NITRIC OXIDE AND HYDROGEN SULFIDE ADMINISTRATION ARE AMONG THE OTHER APPROACHES TO ACTIVATING HIF-1AND GLOBAL HISTONE DEMETHYLATION ON HISTONE SITES, HORMETIC IMPACTS AND POSSIBLY A VERSION OF YOUNGING
The 2018 publication Endogenous hydrogen sulfide regulates histone demethylase JMJD3-mediated inflammatory response in LPS-stimulated macrophages and in a mouse model of LPS-induced septic shock reports: “Overproduction of inflammatory mediators contributes to uncontrolled inflammation during endotoxin shock Cystathionine-γ-lyase (CSE), an enzyme involved in hydrogen sulfide (H2S) biosynthesis, has potential anti-inflammatory activity in a variety of inflammatory diseases. Jumonji domain-containingprotein 3 (JMJD3), a histone 3
Lys27 (H3K27) demethylase, has been implicated in macrophage activation, but its function in CSE-mediated anti-inflammatory activities remains unknown. In the present study CSE was found to be upregulated in macrophages and mouse lipopolysaccharide (LPS) challenge models. LPS stimulation also enhanced the activation of JMJD3 and decreased H3K27me3 levels. JMJD3 knockdown upregulated H3K27me3 levels and attenuated the LPS-mediated inflammatory response. CSE knockout amplified the inflammatory cascade by increasing JMJD3 expression in septic mice. Similarly, enhanced production of inflammatory mediators by macrophages was mitigated by CSE overexpression via inhibition of JMJD3 expression. This is the first report indicating that inflammation enhanced CSE/H2S system biosynthesis, that in turn attenuated the LPS-triggered inflammatory response by regulating JMJD3 expression. Thus, the CSE/H2S system represents an epigenetic-based modification mechanism to prevent uncontrolled inflammation.”
The 2013 publication Nitric oxide modifies global histone methylation by inhibiting Jumonji C domain-containing demethylases reports “Methylation of lysine residues on histone tails is an important epigenetic modification that is dynamically regulated through the combined effects of methyltransferases and demethylases. The Jumonji C domain Fe(II) α-ketoglutarate family of proteins performs the majority of histone demethylation. We demonstrate that nitric oxide ((•)NO) directly inhibits the activity of the demethylase KDM3A by forming a nitrosyliron complex in the catalytic pocket. Exposing cells to either chemical or cellular sources of (•)NO resulted in a significant increase in dimethyl Lys-9 on histone 3 (H3K9me2), the preferred substrate for KDM3A. G9a, the primary methyltransferase acting on H3K9me2, was down-regulated in response to (•)NO, and changes in methylation state could not be accounted for by methylation in general. Furthermore, cellular iron sequestration via dinitrosyliron complex formation correlated with increased methylation. The mRNA of several histone demethylases and methyltransferases was also differentially regulated in response to (•)NO. Taken together, these data reveal three novel and distinct mechanisms whereby (•)NO can affect histone methylation as follows: direct inhibition of Jumonji C demethylase activity, reduction in iron cofactor availability, and regulation of expression of methyl-modifying enzymes. This model of (•)NO as an epigenetic modulator provides a novel explanation for nonclassical gene regulation by (•)NO.”
EXPLAINING HORMESIS
It is interesting that over 10 years ago, I (Vince) became interested in hormesis (Processs through which a mild a and temporary body stress activates a body response leaving the organism healthier), and I wrote a number of blog entries on the topic which remain online. Among the stress activators concerned were heat shock and HIF-1, nitric oxide, and hydrogen sulphide. See these earlier publications, for example. Jim Watson and I even suggested in 2013 that these substances might be served at hormesis health bars. What was not known then was the mechanism of action underlying these hormetic effects, which is herein described as global histome H3K27 me2-3 demethylation via JDJM3 and its close cousin Jumanji domain demethylases. I (Vince) was among the researchers strongly suspecting all along that hormetic effects might be life-extending. I did not appreciate their possible linkage to an actual age-reversal process, however, which Steve Buss and I have now identified as YOUNGING 1.0. In 2013 I generated a blog entry Prospectus for a Grand Unified theory of Biology, Health and Aging in which I asserted that hormesis is so fundamental to biology it will be an important feature of a Grand Unified Therory (GUT) of biology when that emerges. I believe that now even more and that YOUNGING will be part of that GUT.