Press releases and resulting newspaper articles about biomedical research can be misleading, even when they are from the most respectable institutions. The problem is most often with what they leave out. By ignoring a whole stream of prior research by others or parallel current research, they often give the impression that an incremental research result is a fundamental new breakthrough. Such releases are often picked up and republished by hundreds or thousands of newspapers and other publications worldwide. I examine one example of this kind of happening here. And I suggest a few things to keep in mind when reading press releases or newspaper articles about research discoveries.
The press release I will use as an example was dated yesterday and from the Harvard Medical School, not exactly a shabby organization. The press release appeared in whole or part in a great many news publications worldwide. Here it is as seen in Eurekaalert:
“Rare disease reveals new path for creating stem cells — BOSTON, Mass. (November 21, 2010)—As debilitating as disease can be, sometimes it acts as a teacher. — Researchers at Harvard Medical School and the Harvard School of Dental Medicine have found that by mimicking a rare genetic disorder in a dish, they can rewind the internal clock of a mature cell and drive it back into an adult stem-cell stage. This new “stem cell” can then branch out into a variety of differentiated cell types, both in culture and in animal models. — “This certainly has implications for personalized medicine, especially in the area of tissue engineering,” says Bjorn Olsen, the Hersey Professor of Cell Biology at Harvard Medical School and Dean of Research at the Harvard School of Dental Medicine. — These findings appear November 21, online in Nature Medicine. — Fibrodysplasia Ossificans Progressiva (FOP), which affect fewer than 1,000 people worldwide, is a horrific genetic disease in which acute inflammation causes soft tissue to morph into cartilage and bone. Over the course of a few decades, patients gradually become thoroughly ossified, as though parts of their body have turned to stone. There is no cure or treatment. — Damian Medici, an instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, found that, unlike normal skeletal tissue, the pathological cartilage and bone cells from these patients contained biomarkers specific for endothelial cells—cells that line the interior of blood vessels. This led him to question whether or not the cartilage and bone growing in soft tissues of FOP patients had an endothelial origin. — Medici and his colleagues transferred the mutated gene that causes FOP into normal endothelial cells. Unexpectedly, the endothelial cells converted into a cell type nearly identical to what are called mesenchymal stem cells, or adult stem cells that can differentiate into bone, cartilage, muscle, fat, and even nerve cells. (Embryonic stem cells have the potential to become any type of cell, whereas adult stem cells are limited.) — What’s more, through further experiments the researchers found that instead of using the mutated gene to induce the transformation, they could incubate endothelial cells with either one of two specific proteins (growth factors TGF-beta2 and BMP4) whose cellular interactions mimicked the effects of the mutated gene, providing a more efficient way to reprogram the cells. — Afterwards, Medici was able to take these reprogrammed cells and, in both culture dishes and animal models, coax them into developing into a group of related tissue types. — “It’s important to clarify that these new cells are not exactly the same as mesenchymal stem cells from bone marrow,” says Medici. “There are some important differences. However, they appear to have all the potential and plasticity of mesenchymal stem cells.” — “The power of this system is that we are simply repeating and honing a process that occurs in nature,” says Olsen. “In that sense, it’s less artificial than other current methods for reprogramming cells.” — According to study collaborator Frederick Kaplan, Isaac & Rose Nassau Professor of Orthopaedic Molecular Medicine at the University of Pennsylvania School of Medicine and a world expert on FOP, “While we want to use this knowledge to stop the renegade bone formation of FOP, these new findings provide the first glimpse of how to recruit and harness the process to build extra bone for those who desperately need it.” — Medici and Olsen echo this, stating that the most direct application for these findings is the field of tissue engineering and personalized medicine. It is conceivable that transplant patients may one day have some of their own endothelial cells extracted, reprogrammed, and then grown into the desired tissue type for implantation. Host rejection would not be an issue.”
This press release is well written and largely though not completely accurate. However, read even carefully by a person without the right technical background, that person is likely to get certain impressions, including:
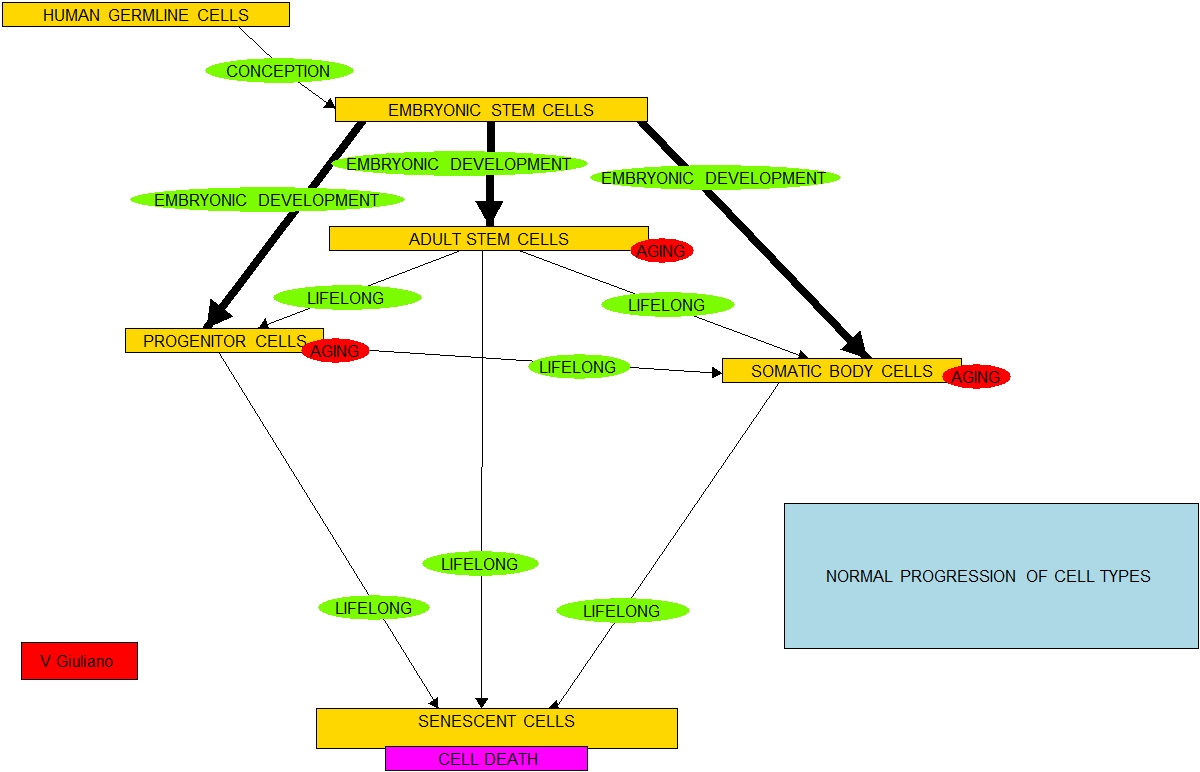
1. The researchers discovered that endothelial cells can revert or be reverted into mesenchymal stem cells (MSCs) or adult stem cells or ones very much like them. The researchers discovered that the clock of cell differentiation can be run backwards.
2. The researchers discovered that reversion of endothelial cells into MSC-like cells can be accomplished by using a mutant version of the FOP gene.
3. The researchers discovered that reversion of endothelial cells into MSC-like cells can also be accomplished using either of two proteins (growth factors TGF-beta2 and BMP4), and this was a more efficient process than using the FOP gene variant.
Of these statements, only the second one is correct. Let’s look at some of the prior research.
It has been known for a long time that endothelial cells can be converted into MSC-like cells
The phenomenon of epithelial-mesenchymal transition has been known to exist for some time; it was not discovered as part of the new research. It is part of developmental biology and is also observed in some cancers. “Epithelial-mesenchymal transition or transformation (EMT) is a program of development of biological cells characterized by loss of cell adhesion, repression of E-cadherin expression, and increased cell mobility. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation(ref).” A few of the many prior papers I have found on the topic are:
(2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. “The epithelial-mesenchymal transition (EMT) is a key developmental program that is often activated during cancer invasion and metastasis. Induction of an EMT in immortalized human mammary epithelial cells (HMLEs) results in the acquisition of mesenchymal traits, but in addition the expression of stem-cell markers– These findings illustrate a direct link between the EMT and the gain of epithelial stem-cell properties.”
(1996) Epithelial-mesenchymal transitions in cancer progression
(1995) Epithelial-mesenchymal transitions in development and tumor progression
EMT has been around and studied as seen in these and many other publications since before the mid 90s and statement #1 above is plain false. Also of course, the whole field of induced pluripotent stem cells (iPSCs) I have written about so often is directly concerned with running the clock of cell differentiation backward. In that field, the reported research is a teaspoon full that is poured into a 50 gallon drum of existing research(ref).
The capabilities of the growth factors TGF-beta2 and BMP4 to induce epithelial-mesenchymal transition have been known for some time
The 2005 paper Transforming Growth Factor-β Signaling during Epithelial-Mesenchymal Transformation: Implications for Embryogenesis and Tumor Metastasis states “–However, more recent studies have implicated a significant role of the transforming growth factor-β (TGF-β) in causing EMT in both development and pathology.”
The 2004 paper Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells also deals with the relationship of the two growth factors to mesenchymal cells. “Thus, signaling cross-talk between BMP and TGF-
TheNovember 2010 paper Low Doses of Bone Morphogenetic Protein 4 Increase the Survival of Human Adipose-Derived Stem Cells Maintaining Their Stemness and Multipotency is another current study relating BMP4 to stem cell maintenance instead of differentiation. “Our results therefore support BMP4 as a promising factor for expanding human adipose tissue-derived MSCs maintaining their properties of stemness and multipotency.”
The 2008 paper BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells reports “We conclude that the activity of BMP4 in EMT during development is recapitulated in adult airway epithelial cells and suggest that this activity may contribute to inflammation and fibrosis in vivo.”
So, statement #3 above is also untrue “The researchers discovered that reversion of endothelial cells into MSC-like cells can also be accomplished using either of two proteins (growth factors TGF-beta2 and BMP4).” This was well known beforehand.
In all fairness, the paper described in the press release did make significant incremental contributions to existing knowledge:
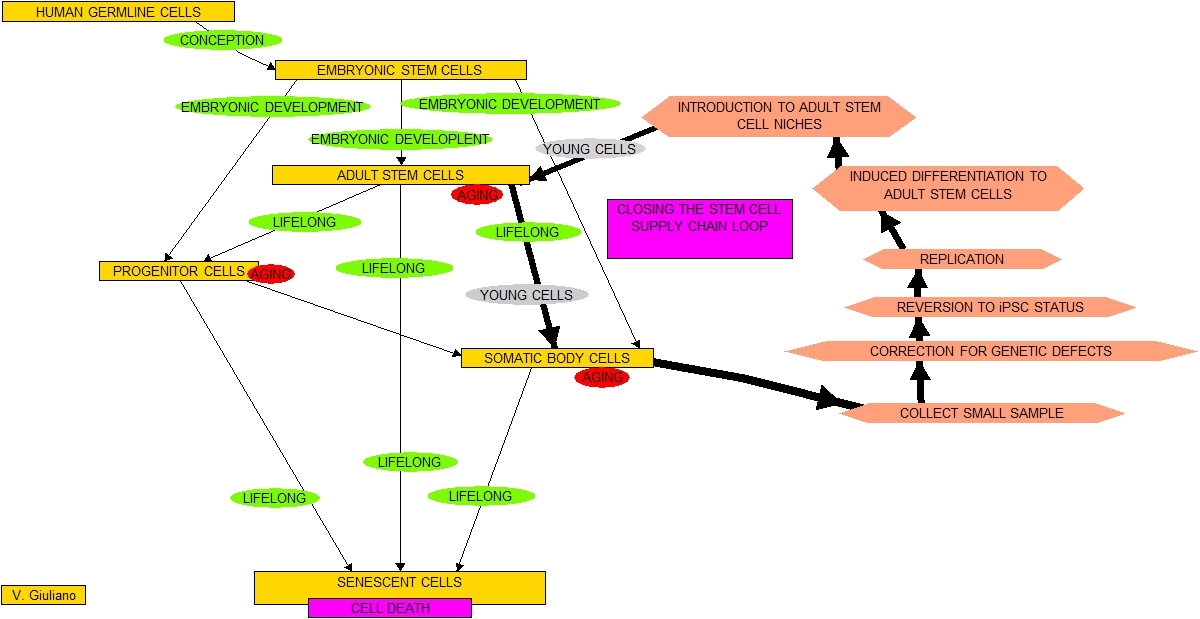
a. The EMT process, well established in biology, can be practically harnessed to create mesenchymal-type adult stem cells.
b. This can be accomplished by using a mutant version of the FOP gene.
c. The authors demonstrated in the laboratory that a more practical approach is to use either TGF-beta2 or BMP4.
My problem is with the press release. I have no dispute with the content of the actual publication in question Conversion of vascular endothelial cells into multipotent stem-like cells. “Mesenchymal stem cells can give rise to several cell types, but varying results depending on isolation methods and tissue source have led to controversies about their usefulness in clinical medicine. Here we show that vascular endothelial cells can transform into multipotent stem-like cells by an activin-like kinase-2 (ALK2) receptor–dependent mechanism. In lesions from individuals with fibrodysplasia ossificans progressiva (FOP), a disease in which heterotopic ossification occurs as a result of activating ALK2 mutations, or from transgenic mice expressing constitutively active ALK2, chondrocytes and osteoblasts expressed endothelial markers. Lineage tracing of heterotopic ossification in mice using a Tie2-Cre construct also suggested an endothelial origin of these cell types. Expression of constitutively active ALK2 in endothelial cells caused endothelial-to-mesenchymal transition and acquisition of a stem cell–like phenotype. Similar results were obtained by treatment of untransfected endothelial cells with the ligands transforming growth factor-β2 (TGF-β2) or bone morphogenetic protein-4 (BMP4) in an ALK2-dependent manner. These stem-like cells could be triggered to differentiate into osteoblasts, chondrocytes or adipocytes. We suggest that conversion of endothelial cells to stem-like cells may provide a new approach to tissue engineering.”
This research, though possibly important, is far from the bottom line when it comes to practical approaches for reverting ordinary body cells into stem cells. See Past blog postings on stem cells and epigenomics for blog entries on many other research efforts aimed at reverting normal cells into stem cells. New research findings related to this subject are now being reported in the literature practically every week.
About reading research press releases or newspaper articles
· Press releases and newspaper articles are prone to building up incremental research findings so they sound like basic breakthroughs. When you see a headline like “Fountain of youth – University of Gokomursk scientists discover how to turn the clock back on skin cells to make stem cells,” be aware that 20 other research groups may have already found ways to do the same thing. The writers don’t like all the nitpicking caveats that the scientists themselves would give. University PR people want to make their institutions look good and newspaper writers like to make their articles simple.
· When reading press releases, particularly ones trumpeting research breakthroughs, be aware of what is left out, perhaps a rich history of past research, perhaps that the discovery being reported is not unique. Perhaps the research being reported is just another large stone in a large edifice still being built. Perhaps it is only a tiny stone. Almost all key discoveries are based on highly-related past discoveries.
· Don’t assume that everything said is accurate. Most press releases and newspaper articles are written by professional writers, people who might not have a good grasp of a technical subject.
· To find the strait scoop on the research, when the press release or newspaper article is triggered by a research publication, go directly to the research publication.
Having said these things press releases and newspaper articles are often very useful because when responsibly written:
· they might convey interesting otherwise-unpublished information based on interviews with the scientists who did the work; they may include fascinating quotes and personal opinions;
· they can explain highly technical developments in plain language understandable to all.
For these reasons, I will continue occasionally to quote from press releases or newspaper articles. However, I will continue to rely for information primarily on the published scientific research literature, on direct interactions with researchers, and on presentations I have seen at respectable research conferences.