By Vince Giuliano and Jim Watson
Immune system functionality declines significantly with advancing age, leading to increased susceptibility to infectious and many other diseases. This is a very important aspect of aging as we know it. This blog entry looks again at the science related this decline and to recent research that may lead tof intervention for halting or reversing it.
BACKGROUND
In large part, this blog entry picks up from the September 2011 blog entry Aging and the immune system – focus on naïve T-cells. If you are not familiar with the topic, we suggest you start by reading that blog before proceeding further here. A central focus of that blog was reversal of thymic involution – age related shrinking and decline of functionality of the thymus gland, This gland makes T cells that are necessary for the operation of the adaptive immune system.
We have two interacting immune systems, the innate immune system which is older and common to more primitive species, a system that offers fixed responses to pathogens and our adaptive immune system which consists of a system of specialized cells and processes that remembers pathogens we encountered earlier and life to enable a rapid and effective defensive response if they are encountered again even years afterward. The 2011 blog focuses on the adaptive immune system and introduces the main cellular actors related to it including T cells, B cells, naïve cells, memory cells, effector cells and thymocytes. It also describes the key role of the thymus gland early in life for processing thymocytes into mature naïve T cells – a role that drops off rapidly after adolescence and is virtually non-existent in older people.” And it covers some possible approaches for regenerating the thymus gland. This present blog covers additional background and more-recent research and insights applicable to that same issue.
MAJOR BOTTOM LINES OF THIS BLOG ENTRY
This blog contains a great deal of detailed information, and it is easy to lose sight of the most important conclusions. So, we list them here.
- Many factors are involved factors in age-related decline in the functioning of the immune systems. Among these is the involution and gradual disappearance of the thymus gland which is key for generating new T cells. But this is only one of several age-related processes related to decline in immune functionality.
- Starting about a dozen years ago, many researchers started to believe that thymic involution could be reversed in older people and functioning of the adaptive immune system might thereby be restored.
- This has been demonstrated to work in mice and dogs.
- There are several possible known approaches for initiating such reversal.
- It has been demonstrated in a clinical trial that administration of human growth hormone (hGH) restores thymic and T cell functionality in individuals infected with HIV. This possibility has been confirmed in other studies, but only in the context of HIV infection.,
- Despite all this, approaches to halting or reversing age-related immune system decline in the translational medicine pipeline appear to be few and far between.
- There are no new clinical trials relating to thymic involution or other approaches for halting or reversing age-related immune system decline on the books. In fact, there can’t be because aging is not officially a disease according to the FDA.
- Most researchers now seem to have lost interest in thymus gland regeneration in healthy aging people – the literature focused directly on this topic was published in 2006 or earlier.
- Some small biotech companies continue to work on the issue. Recombinant human IL-7 is being developed by Cytheris, Inc. right now for the treatment of thymic involution. It may be become FDA approved in the next 2-3 years. A small clinical feasibility trial using hGH may be in the works, according to a personal communication from Gregg Fahey, a long-time experimenter in this field.
- New basic scientific discoveries potentially applicable to immune system regeneration continue to appear. There is new understanding of the molecular biology of thymic involution and thymic regeneration is known to be directly inducible via the FOXN1 transcription factor,
- For individuals wishing to maintain their immune system functionality, there are a few things they can in fact do, some simple and practical, some more extreme. These are based on knowledge of many factors that can accelerate or slow down the age-related decline in functional immunity.
- From a science viewpoint, the prospectus for partially or reversing age-related immune senescence is very good. It may be some time before practical and economic barriers that are stopping this from happening are overcome, however.
AGE-RELATED DECLINE IN THE INNATE IMMUNE SYSTEM
Athough immunosenescence is primarily a T cell problem and our central focus here is on the adaptive immune system, there is also related decline in the functionality of the innate immune system and many issues there that come with aging. The innate immune system includes neutrophils, monocytes/macrophages, microglia, dendritic cells, and natural killer cells. Here are the major aging-related impacts on the innate immune system:
A. Neutrophil changes– Neutrophils become less functional and undergo a lot of changes with aging. There may be a preserved neutrophil number or there can be a decrease in neutrophil number. Either way, they don’t work as well. There are major problems with decreased chemotaxis decreased opsonization, decreased phagocytosis, and decreased free radical production (peroxisomes). There is also a decrease in signaling molecule expression (GM-CSF-R, TLR-4, fMLP-R, TREM-1). There are also changes to the plasma membranes, including a reduced levels of cholesterol, increased membrane fluidity, dysregulation of receptor recruitment to lipid rafts, decreased receptor signaling (KPB, JAK, PI3K). There is a preserved expression of adhesion molecules however.
B. Monocyte/Macrophage changes– There is a clear INCREASE in the CD16+ monocyte/macrophage population with aging. This is a “pro-inflammatory phenotype”. There is also a decrease in antigen presentation and a decrease in phagocytic ability of the mononuclear cells. They also cannot make as many free radicals.
C. Dendritic Cell (DC) changes– Dendritic cells clearly decline in number with aging. They make less IL-12, which is an anti-inflammatory cytokine. There is also a decrease in the subpopulation of TLR-7 or TLR-9 cells.
D. Natural Killer cell (NK) changes– Overall increase in percentage and absolute NK cell number. There is an increase in some NK cell populations and a decrease in other NK cell populations, but overall, the absolute number increases with aging. However, there is a decrease in function of NK cells with aging. There is clearly a decrease in CD56+ subpopulation, which is an immunoregulatory subtype. However, there is an increase in the CD56- subpopulation, which are the cytotoxic NK cells. There is a preserved production of IFN-gamma. There is a decrease in chemokine production.
Age-related changes in some of these cell types can also impact on the adaptive immune system. For example, dendritic cells serve for communication between the innate and adaptive immune systems, and can activate lymphocytes and initiate other activities of the adaptive immune system.
AGE-RELATED DECLINE IN THE ADAPTIVE IMMUNE SYSTEM
The decline in functionality of the adaptive immune system is mainly dueto decline in numbers of naive T cells corresponding to increase in numbers of memory T cells.
Put very simply: relatively early in life the thymus makes what is supposed to be a lifelong supply of naïve T cells out of precursor cells in the bone marrow. When those naïve T cells encounter a foreign invader such as many bacteria, viruses or cancer cells, they convert to being memory cells which can recognize the same invader and speed up the defense against it later in life. The thymus essentially begins to shut down after adolescence (a process called thymic involution), meaning that naïve T cells can continue to divide but are no longer generated in the thymus. So as people age the population of naïve T cells become smaller and smaller and older and the population of memory cells becomes larger and larger reflecting the pathogens that have been encountered. More exactly:
“A naïve T cell or Th0 cell[1][2][3] is a T cell that has differentiated in bone marrow, and successfully undergone the positive and negative processes of central selection in the thymus. Among these are the naive forms of helper T cells (CD4+) and cytotoxic T cells (CD8+). A naive T cell is considered mature and unlike activated T cells or memory T cells it has not encountered its cognate antigen within the periphery(ref).”
“Memory T cells are a subset of infection– as well as potentially cancer-fighting T cells (also known as a T lymphocyte) that have previously encountered and responded to their cognate antigen; thus, the term antigen-experienced T cell is often applied. Such T cells can recognize foreign invaders, such as bacteria or viruses, as well as cancer cells. Memory T cells have become “experienced” by having encountered antigen during a prior infection, encounter with cancer, or previous vaccination. At a second encounter with the invader, memory T cells can reproduce to mount a faster and stronger immune response than the first time the immune system responded to the invader. This behaviour is utilized in T lymphocyte proliferation assays, which can reveal exposure to specific antigens(ref).”
Age-related decline in thymic function
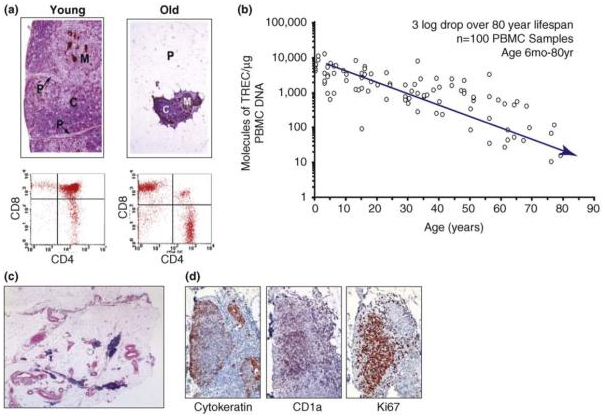
This diagram from the 2009 publication Thymic involution and immune reconstitution tells the story:
“Thymic function decreases with age. (a) Top panel shows cytokeratin-immunostained and hematoxylin & eosin (H&E)-counterstained thymus sections from healthy young and aged human donors. In the aged thymus, the perivascular space (P) is increased in size and the thymopoietic cortex (C) and medulla (M) are significantly constricted. Lower panel shows flow cytometric analysis of CD4 and CD8 on thymocytes isolated from healthy young and aged human thymus donors; a clear reduction in double positive (DP) CD4+CD8+ T cells be seen in aged individuals. (b) sjTREC (single joint T-cell receptor excision circle) measurement per microgram of circulating peripheral blood mononuclear cell (PBMC) DNA from healthy human donors ranging from <1 to ∼80 years of age demonstrates a steady decline of naïve T-cell output with age. (c) Human thymus tissue from a 78-year-old female. Tissue was immunostained with anti-cytokeratin and H&E counterstained to show remaining islands of cortical and medullary tissue (blue). (d) Islands of cortical and medullary tissue in aged thymus show signs of active thymopoiesis by CD1a and Ki67 immunostain.”
Thymic involution: “One of the major characteristics of vertebrate immunology is thymic involution, the shrinking of the thymus with age, resulting in changes in the architecture of the thymus and a decrease in tissue mass.[1] This process is a conserved sequence or (orthologous sequences) in almost all vertebrates, from birds, teleosts, amphibians to reptiles, though the thymus of a few species of sharks are known not to involute.[1][2] T-cells are named for the thymus where T-lymphocytes migrate from the bone marrow to mature. Its regression has been linked to the reduction in immunosurveillance in the elderly.[3] Though thymic involution has been linked to senescence, it is not induced by senescence as the organ starts involuting from a young age [4] – as early as the first year of life in humans(ref).[5] ”
“The ability of the immune system to mount a strong protective response depends on the receptor diversity of naive T-cells (TCR). Thymic involution results in a decreased output of naïve T lymphocytes – mature T cells that are tolerant to self antigens, responsive to foreign antigens, but have not yet been stimulated by a foreign substance. In adults, naïve T-cells are hypothesized to be primarily maintained through homeostatic proliferation, or cell division of existing naïve T cells. Though homeostatic proliferation helps sustain TCR even with minimal to nearly absent thymic activity, it does not increase the receptor diversity.[11] For yet unknown reasons, TCR diversity drops drastically around age 65.[11] Loss of thymic function and TCR diversity is thought to contribute to weaker immunosurveillance of the elderly, including increasing instances of diseases such as cancers, autoimmunity, and opportunistic infections(ref).”
Quoting further from the earlier blog entry “One of the hallmark of advanced aging is weakening adaptive immune systems, often referred to as immunosenescence. “These age-associated immune dysfunctions are the consequence of declines in both the generation of new naïve T and B lymphocytes and the functional competence of memory populations(ref).” This decline appears to be due to a variety of causes. For one matter, thymic involution starts at an early age. “The thymus begins to shrink (atrophy) after adolescence. By middle age it is only about 15% of its maximum size(ref). There are strong age-related changes in hormone production(ref). Older people may take anti-inflammatory medications like prednisone which inhibit immune function. And changes in patterns of epigenetic markers alter gene activation so as to reduce responsiveness of T cells with age.”
“In particular, homeostatic mechanisms related to naïve T-cells tend to become deregulated with advancing age in primates and humans. A 2011 report Age-related deregulation of naive T cell homeostasis in elderly humansconcludes “Our results show that lower naive T cell numbers were associated with a lower thymic function and higher activation and proliferating naive T cell levels. We then analyzed sjTREC numbers and relative telomere length from sorted naive T cells. Our results show that the aberrant activation and proliferation status was related to lower sjTREC numbers (a peripheral proliferation marker) and both, higher CD57 expression levels and shortened telomeres (replicative senescence-related markers). Elderly individuals show a greater contraction of the CD8 naive T cell numbers and all homeostatic alterations were more severe in this compartment. In addition, we found that low functional thymus show a CD4-biased thymocyte production. Taken together, our results suggest a homeostatic deregulation, affecting mostly the naive CD8 T cell subset, leading to the accumulation of age-associated defects in, otherwise, phenotypically naive T cells.”
FACTORS RELATED TO FUNCTIONAL DECLINE IN ADAPTIVE IMMUNITY
These can be many, including problems with the haematopoietic precursor bone marrow cells, problems in the thymus, and existence of various triggers for thymic involution including sex hormones and certain diseases.
Impaired hematopoietic stem cells
A dramatic change in the immune system with aging is in the naive T cell compartment. Part of this is due to the inability to generate lymphoid progenitors by functionally impaired haematopoietic stem cells, the bone marrow cells that are processed by the thymus to make naïve T cells.. This impaired hematopoietic stem cell problem has been explained to be due to a deficiency in the capacity to repair DNA damage.
The 2007 publication Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age reports “A diminished capacity to maintain tissue homeostasis is a central physiological characteristic of ageing. As stem cells regulate tissue homeostasis, depletion of stem cell reserves and/or diminished stem cell function have been postulated to contribute to ageing. It has further been suggested that accumulated DNA damage could be a principal mechanism underlying age-dependent stem cell decline. We have tested these hypotheses by examining haematopoietic stem cell reserves and function with age in mice deficient in several genomic maintenance pathways including nucleotide excision repair, telomere maintenance and non-homologous end-joining. Here we show that although deficiencies in these pathways did not deplete stem cell reserves with age, stem cell functional capacity was severely affected under conditions of stress, leading to loss of reconstitution and proliferative potential, diminished self-renewal, increased apoptosis and, ultimately, functional exhaustion. Moreover, we provide evidence that endogenous DNA damage accumulates with age in wild-type stem cells. These data are consistent with DNA damage accrual being a physiological mechanism of stem cell ageing that may contribute to the diminished capacity of aged tissues to return to homeostasis after exposure to acute stress or injury.” Other publications from the same 2007-2008 era with similar themes include:
- Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment.
- Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging.
- DNA repair is limiting for haematopoietic stem cells during ageing.
- Hematopoietic stem cells and the aging hematopoietic system.
There are many more-recent articles relating to the functionality of aging and inadequate DNA repair such as can be found in this list. We comment that recent research suggests that a limiting factor in DNA repair in older people may be inadequate nuclear supplies of NAD+ — a situation that might be addressed in older people by supplementation with a NAD+ precursor such as nicotinamide riboside or nicotinamide mononucleotide. Because that subject is part of a larger picture of aging also involving the mitochondria, metabolism and ROS, we will deal with the intricacies of it in a separate blog entry that should be published shortly.
Thymic involution
This is a process that starts at age 1 and results in complete involution by 60 years of age for most people. As a result, 80% of the lymphocyte pool is depleted. This dramatically impairs the adaptive immune system’s capability to respond to new antigens. This decrease is seen in lymphoid organs and in peripheral blood. It is more pronounced in CD8+ than CD4+ cells. For CD4+ cells, the CD31 surface antigen correlates with naive status. The newly emigrated thymic CD4+ cells are CD4+CD31+. With aging, this population declines in a direct correlation with thymic involution.
Again from the earlier blog entry: ‘The thymus is also extremely susceptible to diseases. Malnutrition, nutritional imbalances and other triggers of thymic involution. See The thymus is a common target organ in infectious diseases(2006) and The thymus is a common target in malnutrition and infection (2007). The 2010 publication Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responsesreports “The thymus gland, where T lymphocyte development occurs, is targeted in malnutrition secondary to protein energy deficiency. There is a severe thymic atrophy, resulting from massive thymocyte apoptosis (particularly affecting the immature CD4+CD8+ cell subset) and decrease in cell proliferation. The thymic microenvironment (the non-lymphoid compartment that drives intrathymic T-cell development) is also affected in malnutrition: morphological changes in thymic epithelial cells were found, together with a decrease of thymic hormone production, as well as an increase of intrathymic contents of extracellular proteins. Profound changes in the thymus can also be seen in deficiencies of vitamins and trace elements. Taking Zn deficiency as an example, there is a substantial thymic atrophy. Importantly, marginal Zn deficiency in AIDS subjects, children with diarrhoea and elderly persons, significantly impairs the host’s immunity, resulting in an increased risk of opportunistic infections and mortality; effects that are reversed by Zn supplementation. Thymic changes also occur in acute infectious diseases, including a severe thymic atrophy, mainly due to the depletion of CD4+CD8+ thymocytes, decrease in thymocyte proliferation, in parallel to densification of the epithelial network and increase in the extracellular matrix contents, with consequent disturbances in thymocyte migration and export. In conclusion, the thymus is targeted in several conditions of malnutrition as well as in acute infections. These changes are related to the impaired peripheral immune response seen in malnourished and infected individuals. Thus, strategies inducing thymus replenishment should be considered as adjuvant therapeutics to improve immunity in malnutrition and/or acute infectious diseases.”
Factors that accelerate the functional decline in Adaptive Immunity
CMV viral infections appear to be one the biggest factors that accelerates immune decline. Oxidative stress appears to be another major cause. The administration of sex steroid exogenous hormone replacement therapy also hastens immune aging. Smoking is another factor that accelerates immune senescence. “Among additional triggers for thymic involution are the presence of beta catenin, an oncogene(ref) and the presence of T-cell lymphoma(ref)(ref).”
Sex hormones and the thymus
It appears that sex hormones are implicated in thymic involution and that for males castration may be an effective anti-aging strategy, not that we recommend it. See for example the 2008 article The role of sex steroids and gonadectomy in the control of thymic involution. You can also check out articles in this list that relate hormones to thymic finctionality. Preservation of thymic activity may explain why Korean court eunuchs may have lived so long.. “They found that the average life span for a Korean court eunuch was about 70 years, plus or minus 1.76 years. That was 14.4 to 19.1 years longer than their average non-eunuch contemporaries, who tended to live between 50.9 and 55.6 years(ref)”. We note that this is a greater life span lengthening effect than any other intervention known in humans so far.
Decline in hGH and increases in sex steroids and glucocorticoids cause thymic involution => decline in Naive cells and increase in Memory cell senescence
Some authors have stated that involution of the mammalian thymus is one of the leading regulators of aging. In 1985, Steinman showed that thymic function actually starts to decline in the first year of life, but the dramatic involution of the thymus starts during puberty and is clearly driven by sex steroids. The block occurs at the TN1 to TN2 stage in the thymocyte differentiation of stromal cells within the thymus. These cells are identified by surface markers (CD3, CD4, and CD8). Development through these early stages is dependent on IL-7. However other important cytokines also stimulate thymopoiesis, such as keratinocyte growth factor (KGF), GM-CSF,
However, it appears that while blocking sex hormones may delay thymic involution, it cannot prevent it from eventually happening. The2005 publication Reassessing the role of growth hormone and sex steroids in thymic involution reports “The concomitant decline in growth hormone (GH) and increase in sex steroid production with age is thought to be responsible for thymic involution. If changes in the production of these hormones trigger or sustain thymic involution, that process should be accelerated in little mice, which have a genetic deficiency resulting in reduced production of thymopoietic GH, and delayed in the hypogonadal strain, which fails to produce thymocytotoxic sex steroids. The results indicated that thymic involution in both strains progressed in a manner similar to their normal littermates. That blocking sex steroid production did not delay thymic involution was surprising since castration reportedly increases thymus cellularity. Re-examination of that phenomenon revealed that, while gonadectomy results in increased thymus size, its effects are transient, and the thymus ultimately undergoes involution. Taken together, these data suggest that age-related changes in the endocrine system do not underlie thymic involution.”
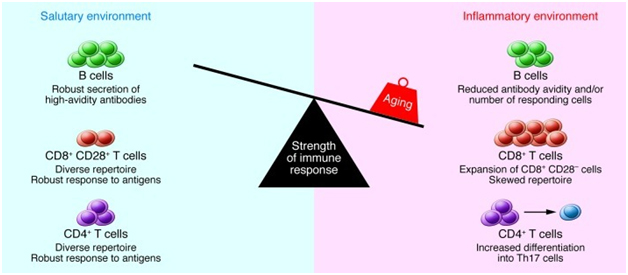
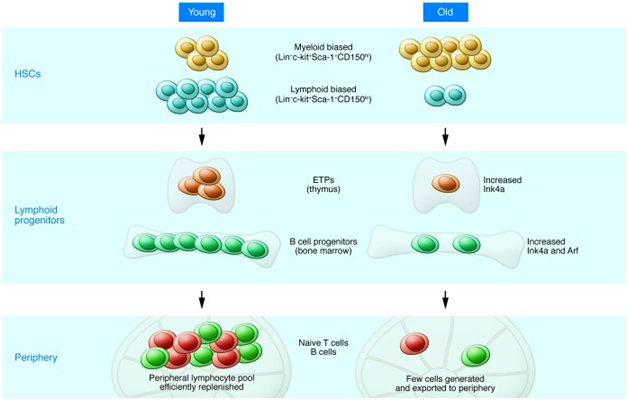
A 2013 publication Causes, consequences, and reversal of immune system agingoutlines additional age-related changes in adaptive immune function in addition to thymic involution as shown in these two diagrams:
“The strength of the immune response declines with age: Multiple age-related changes can affect the composition and function of lymphocytes in secondary lymphoid tissues. CD4+ Th cells exhibit activation defects and increased differentiation into Th17 cells. CD8+ T cells undergo an oligoclonal expansion and loss of CD28 in humans and exhibit impaired function. The number of B cells that respond to influenza is reduced, and antibody avidity in response to carbohydrate antigens is diminished. In addition, the tissue environment includes an increased concentration of inflammatory cytokines, which may be produced by stromal elements, dendritic cells, or aging B and T cells. The increased number of memory cells that occupy tissue niches and the inflammatory milieu in turn may compromise the ability of naive B and T cells migrating from the bone marrow and thymus to lodge in the tissue. Together, these changes result in diminished immune function in the elderly. “
“Effects of aging on HSCs and lymphocyte progenitors: Lymphopoiesis in the young (left) is characterized by robust B and T cell production in the bone marrow and thymus. The pool of HSCs includes a relatively high number of lymphoid-biased stem cells that efficiently generate lymphoid progenitors with high proliferative potential. However, with increasing age (right), the number of lymphoid-biased HSCs declines and myeloid-biased stem cells predominate, contributing to the reduced numbers of lymphoid progenitors. In addition, B cell progenitors in the bone marrow and T cell progenitors in the thymus exhibit reduced rates of proliferation and higher levels of apoptosis compared with their young counterparts. The increased expression of Ink4a and Arf in pro-B cells and Ink4a in ETPs contribute to this decreased proliferation/increased apoptosis. The decline in primary lymphopoiesis in turn results in a reduced number of naive cells that migrate to secondary lymphoid tissues such as the spleen. “
Reversing thymic involution as a strategy for restoring age-related decline in immune function
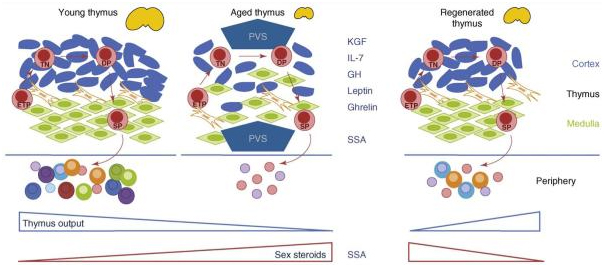
There appears to be wide agreement that thymic involution can be halted or reversed, This topic was treated in the earlier blog entry and again is the focus here. This diagram is also from the 2009 publication Thymic involution and immune reconstitution.
In part, the legend states “Treatment with thymostimulatory cytokines, such as keratinocyte growth factor (KGF), interleukin 7 (IL-7), growth hormone (GH), leptin and ghrelin, or sex steroid ablation therapy (SSA), can promote regeneration of an atrophic thymus—increasing total cellularity, restoring thymic architecture, increasing output of naïve T cells and rejuvenating the diversity of the peripheral TCR repertoire. This might occur by several mechanisms including an increase in thymus seeding by early T-lineage progenitors (ETPs) or increased proliferation and/or differentiation of triple negative (TN), double positive (DP) or single positive (SP) thymocytes.” That document also reports “Chronic thymus involution associated with aging results in less efficient T-cell development and decreased emigration of naïve T cells to the periphery. Thymic decline in the aged is linked to increased morbidity and mortality in a wide range of clinical settings. Negative consequences of these effects on global health make it of paramount importance to understand the mechanisms driving thymic involution and homeostatic processes across the lifespan. There is growing evidence that thymus tissue is plastic and that the involution process might be therapeutically halted or reversed. We present here progress on the exploitation of thymosuppressive and thymostimulatory pathways using factors such as keratinocyte growth factor, interleukin 7 or sex steroid ablation for therapeutic thymus restoration and peripheral immune reconstitution in adults.”
Strategies for slowing, halting or reversing thymic involution
A substantial body of research literature has emerged suggesting strategies for slowing, halting or reversing thymic involution. See the articles in this list. Some of these approaches were reviewed in the earlier blog entry. Here, we will focus mainly on more recent research, such as related to administration of the transcription factor Foxn1, and on use of human growth factor hGH..
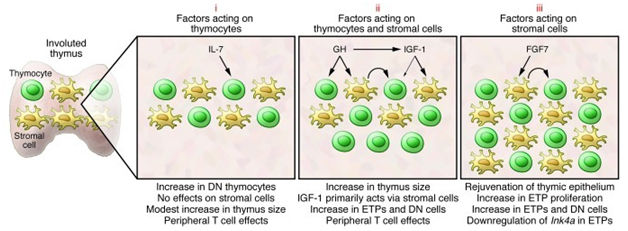
Referring again to the publication Causes, consequences, and reversal of immune system aging, the situation as viewed in March 2013 can be summarized in this diagram.
“Selected strategies to rejuvenate the involuted thymus. The potential of several hormones and growth factors to rejuvenate the involuted thymus has been tested in various preclinical and clinical trials. Many of these factors can be grouped into three categories. Those in the first (i), such as IL-7, bind to progenitors in the bone marrow and thymus and have only modest effects on thymopoiesis. There is little evidence that IL-7 has effects on thymic stromal cells. Instead, the benefit of IL-7 may lie in its ability to stimulate peripheral T cell survival/expansion. The second category (ii) includes hormones such as GH that have been demonstrated in preclinical and clinical trials to stimulate thymopoiesis and increase thymic size. Many GH effects are mediated through induction of IGF-1. IGF-1 can bind to receptors on thymic stroma and thymocytes, although its actions are primarily mediated through effects on the former cells. Stromal cell–derived factors presumably then act on thymocytes (curved arrow). A third category of factors (iii), typified by FGF7, bind to stromal cells but not thymocytes. Stromal cell–induced factors then act on thymocytes (curved arrow), and we have recently demonstrated that effects include downregulation of Ink4a in ETPs (82). The thymopoietic effects of several additional factors have been evaluated, and recent detailed reviews should be consulted for more information (99, 100).”
The key role of FOXN1
Recent mouse-level studies indicate that a single transcription factor FOXN1 can both halt thymic involution and initiate regeneration of an involuted thymus. The FOXN1 gene exists in mice and humans and FOXN1 knockout mice have been used to study cancer and skin issues. More recently, a number of studies have appeared that relate FOXN1 to thymus gland development and regeneration(ref)(ref)(ref)
The 2011 publication relates to aged miceOverexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. “The forkhead box n1 (Foxn1) transcription factor is essential for thymic organogenesis during embryonic development; however, a functional role of Foxn1 in the postnatal thymus is less well understood. We developed Foxn1 transgenic mice (Foxn1Tg), in which overexpression of Foxn1 is driven by the human keratin-14 promoter. Expression of the Foxn1 transgene increased the endogenous Foxn1 levels. In aged mice, overexpression of Foxn1 in the thymus attenuated the decline in thymocyte numbers, prevented the decline in frequency of early thymic progenitors, and generated a higher number of signal joint TCR excised circle. Histologic studies revealed that structural alterations associated with thymic involution were diminished in aged Foxn1 Tg. Total numbers of EpCAM+ MHC II+ and MHC II(hi) thymic epithelial cells were higher in young and old Foxn1Tg and more EpCAM+ MHC II(hi) TEC expressed Ki-67 in aged Foxn1Tg compared with WT. Furthermore, Foxn1Tg displayed a significant reduction in the expansion of splenic CD4+ memory compartments and attenuated the decline in CD4+ and CD8+ naive compartments. Our data indicate that manipulation of Foxn1 expression in the thymus ameliorates thymopoiesis in aged mice and offer a strategy to combat the age-associated decline in naive T-cell production and CD4 naive/memory ratios in the elderly.
The 2013 publication Enhancing T lineage production in aged mice: a novel function of Foxn1 in the bone marrow nichereports “Foxn1 is essential for thymic organogenesis and T lymphopoiesis. Whereas reduced Foxn1 expression results in a decline in T lymphopoiesis, overexpression of Foxn1 in the thymus of a transgenic mouse model (Foxn1Tg) attenuates the age-associated decline in T lymphopoiesis. T lymphopoiesis begins with early T cell progenitors (ETP), derived from multipotent progenitors (MPP) in the bone marrow (BM). A decline in MPP and ETP numbers with age is thought to contribute to reduced T lymphopoiesis. Previously, we showed that reduced ETP number with age is attenuated in Foxn1 transgenic (Tg); whether the effect is initiated in the BM with MPP is not known. In this study, we report that Foxn1 is expressed in wild-type BM and overexpressed in Foxn1Tg. With age, the number of MPP in Foxn1Tg was not reduced, and Foxn1Tg also have a larger pool of hematopoietic stem cells. Furthermore, the Foxn1Tg BM is more efficient in generating MPP. In contrast to MPP, common lymphoid progenitors and B lineage cell numbers were significantly lower in both young and aged Foxn1Tg compared with wild type. We identified a novel population of lineage(neg/low), CD45(pos) EpCAM(pos), SCA1(pos), CD117(neg), CD138(neg), MHCII(neg) cells as Foxn1-expressing BM cells that also express Delta-like 4. Thus, Foxn1 affects both T lymphopoiesis and hematopoiesis, and the Foxn1 BM niche may function in skewing MPP development toward T lineage progenitors.
Finally, the 2014 publicationRegeneration of the aged thymus by a single transcription factor reports: “Thymic involution is central to the decline in immune system function that occurs with age. By regenerating the thymus, it may therefore be possible to improve the ability of the aged immune system to respond to novel antigens. Recently, diminished expression of the thymic epithelial cell (TEC)-specific transcription factor Forkhead box N1 (FOXN1) has been implicated as a component of the mechanism regulating age-related involution. The effects of upregulating FOXN1 function in the aged thymus are, however, unknown. Here, we show that forced, TEC-specific upregulation of FOXN1 in the fully involuted thymus of aged mice results in robust thymus regeneration characterized by increased thymopoiesis and increased naive T cell output. We demonstrate that the regenerated organ closely resembles the juvenile thymus in terms of architecture and gene expression profile, and further show that this FOXN1-mediated regeneration stems from an enlarged TEC compartment, rebuilt from progenitor TECs. Collectively, our data establish that upregulation of a single transcription factor can substantially reverse age-related thymic involution, identifying FOXN1 as a specific target for improving thymus function and, thus, immune competence in patients. More widely, they demonstrate that organ regeneration in an aged mammal can be directed by manipulation of a single transcription factor, providing a provocative paradigm that may be of broad impact for regenerative biology.”
There are many issues connected with non-thymic actions of FOXN1, and questions as to whether the mouse finding can be applied to thymus regeneration in humans. The 2012 publication Insights on Foxn1 Biological Significance and Usages of the “Nude” Mouse in Studies of T-Lymphopoiesisrelates: “FoxN1 is a transcription factor whose functions are executed by targeting other genes through its DNA binding domain. Therefore, to understand its functional mechanisms in determining its target genes is important. However, the precise target genes that are regulated by FoxN1 remain ill defined, mostly due to technical difficulties in precisely isolating enough physiologically intact TECs at certain developmental stages. — Recent progress using advanced technology to study FoxN1‘s roles in the thymus shows that FoxN1 regulates not only TEC patterning in the fetal stage but also TEC homeostasis in the postnatal thymus. Comparing the thymus with the skin, FoxN1 has its own distinct roles and impacts on organs in the generation and maintenance of three-dimensional microstructure and pigmentation, respectively. FoxN1‘s role in the neuron has been brought up, but is still obscure. There is still plenty of room to apply nude and secondary nude (conditional FoxN1 gene knockout) mouse models in studies of immunology, hematology, and tumorgenesis. The functional mechanisms of FoxN1‘s collaborative roles with other genes during thymic development and aging remain to be further determined.”
Other publications related to FOXN1 and the thymus are
- Deletion of FoxN1 in the thymic medullary epithelium reduces peripheral T cell responses to infection and mimics changes of aging. (2012)
- Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner (2009)
My assessment is that it will be some time before a FOXN1 therapy for reversing human thymic involution reaches the clinical trial stage, if ever. We are more likely to see a clinical trial relating to FOXN1 as a therapy for averting immunosenescence in HIV patients. Reversing thymic involution would still be the trial target, but the context for testing would be limited to HIV-infected people.
Human growth hormone treatment for reversing thymic involution
The use of growth hormone to restore thymic functionality in animal models goes back to 1986, when the implantation of pituitary adenoma cells secreting GH into rats was seen to induce thymic growth. See the publication GH3 pituitary adenoma cells can reverse thymic aging in rats. The following year, growth hormone treatment in dogs was shown to increase thymulin production. See Growth hormone treatment stimulates thymulin production in aged dogs. In 2003 Gregg Fahey reported partial thymic regeneration due to hGH administration in a single human being (thought to be himself)(ref). .
IGF-1 is induced by hGH
The hormone that actually induces thymulin is IGF-1, which is produced by the liver in response to hGH release from the pituitary. Such hGH release is thought to be the major reason why the hormone thymulin declines with aging. GH induces both bone marrow rejuvenation and thymic rejuvenation. This effect on the bone marrow is normally mediated by CSF-1 and IL-3, which both induced expression and synthesis of IGF-1 locally within the bone marrow by bone marrow cells. However, adding exogenous IGF-1 to bone marrow cells decreases apoptotic cell death by 50%. See the 1996 document Growth Hormone, Growth Factors and Hematopoiesis.
Safety of hGH or IGF-1 treatment
hGH administration is more often thought to shorten rather than lengthen lilespans(ref)(ref)(ref).
The reader may recall that in many of our blog entries we have discussed the Dr. Jekyll and Mr, Hyde nature of IGF-1. On the one hand it is essential for growth and wound healing. On the other hand, IGF-1 expression promotes many disease processes and hallmarks of aging. There seems to be a lot of agreement that to slow or prevent aging, it is desirable to minimize IGF-1 expression and therefore not induce it with hGH. In fact, you want to suppress expression of IGF-1. See the blog entry Longevity and the GH–IGF Axis. Therefore it might seem that using hGH for thymic regeneration may involve both life extending and life-shortening aspects. It could be, however, that hGH induces a biphasic response curve and that the low dose required for thymic regeneration is safe and beneficial.
Clinical trials of hGH for reversing thymic involution
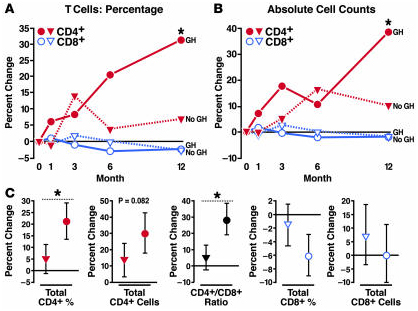
A highly relevant clinical trual Growth Hormone to Increase Immune Function in People With HIV was initiated in 2003 by the National Institutes of Allergy and Infectuous Diseases. As reported in the 2008 publication Growth hormone enhances thymic function in HIV-1-infected adults: “Growth hormone (GH) is an underappreciated but important regulator of T cell development that can reverse age-related declines in thymopoiesis in rodents. Here, we report findings of a prospective randomized study examining the effects of GH on the immune system of HIV-1-infected adults. GH treatment was associated with increased thymic mass. In addition, GH treatment enhanced thymic output, as measured by both the frequency of T cell receptor rearrangement excision circles in circulating T cells and the numbers of circulating naive and total CD4(+) T cells. These findings provide compelling evidence that GH induces de novo T cell production and may, accordingly, facilitate CD4(+) T cell recovery in HIV-1-infected adults. Further, these randomized, prospective data have shown that thymic involution can be pharmacologically reversed in humans, suggesting that immune-based therapies could be used to enhance thymopoiesis in immunodeficient individuals.”
“Comparison of changes in the GH arm versus the observational control arm over the first year of the study showed that GH treatment was associated with significant increases in the percentage (A) and absolute count (B) of CD4+ T cells (red). (C) Comprehensive regression analysis, including crossover data of GH treatment in observational controls, showed that GH treatment (circles) was associated with significant increases in CD4+ T cell percentage and the CD4+/CD8+ T cell ratio when compared with no GH. Increases in the absolute count of CD4+ T cells trended toward statistical significance in this analysis. There were no remarkable GH-associated changes in the percentage or absolute count of CD8+ T cells. Estimated changes with 95% CIs are shown. Regression analysis estimated the effects of 1 year of GH treatment compared with changes over 1 year in the absence of GH. Median values are shown in A and B. CIs and additional data are shown in Tables Tables22 and and3.3. *P < 0.05 for comparison of GH versus no GH.”
A subsequent 2013 publication Low-dose growth hormone for 40 weeks induces HIV-1-specific T cell responses in patients on effective combination anti-retroviral therapyreports: “Recombinant human growth hormone (rhGH) administered to combination anti-retroviral therapy (cART)-treated human immunodeficiency virus-1 (HIV-1)-infected individuals has been found to reverse thymic involution, increase total and naive CD4 T cell counts and reduce the expression of activation and apoptosis markers. — Here we report that administration of low-dose rhGH over 40 weeks with effective cART resulted in greater improvement of T lymphocyte function than observed with cART alone, and provide further evidence that such an approach could also reduce levels of immune activation. “
Do we think that the positive benefits seen in HIV-infected people would also show up in older people who have age-related thymic involution?
In short, yes. Why not?
So,research on reversal of decline in thymus and lymphocyte function via hGH administration seems to be alive and well in the subdomain of treatment of patients infected with HIV. Not so in the case of aging. The initial mouse literature on use of GH for reversal of thymic involution go back to 2002 and literature discussions of use on HGH as an effective human immune-extending therapy seem to have petered off around 2006. There seems to be no more-recent research literature related directly to hHG administration for reversing thymic involution in normal aging individuals – although this idea continues to be discussed in some anti-aging blogs.
Based on private communications, I think there may be a small feasibility clinical trial related to hGH restoration of aging involuted thymic glands proposed by a biotech company in the near future. Other than for that, I do not forsee much real action here. The issue is that FDA-authorized clinical trials can only be for disease conditions, and aging is not considered to be a disease.
The rest of this document is concerned with additional factors that might contribute to thymic involution or reconstitution, and with some possible practical applications of these.
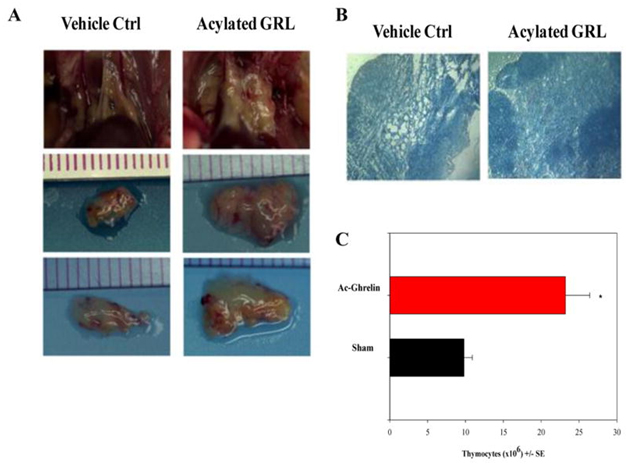
- Ghrelin – In 1999, an endogenous ligand for the previously discovered “growth hormone secretagogue receptor” (GHS-R) was discovered. This molecule is now called Ghrelin and activates the GHS-R, which is a specific G protein-coupled receptor. Ghrelin is commonly called “the hunger molecule.” See the blog entry Ghrelin, hunger, obesity and aging. There is both an acylated ghrelin and a des-acyl form of gherkin circulating in the plasma. The des-acyl ghreline does not bind to GHS-R1a, but both the acylated and des-acyl form binds to common sites on cardiomyocytes and endothelial cells. They work similarly to IGF-1 to prevent apoptosis in cells. Ghrelin is able to strongly stimulate the release of hGH from the pituitary by binding to the GHS-R. Ghrelin is produced by the stomach, but it is also produced elsewhere in the body. Ghrelin inhibits leptin and induces hunger. Ghrelin is actually produced by human T cells, monocytes, and dendritic cells. Ghrelin has potent inhibitory effects on pro-inflammatory cytokine production by activated immune cells, such as T cells, monocytes, and dendritic cells. Ghrelin significantly increases the cellularity of the thymus in older mice. Ghrelin also increased the engraftment of a bone graft. Ghrelin looks like a promising new approach to stimulating the thymus.
Activated ghrelin increases thymic size and cellularity in aging mice. Reference and Image source: Rejuvenation of the Aging Thymus: Growth Hormone- and Ghrelin-Mediated Signaling Pathways
- 2. Gonadectomy – We have already mentioned castration and the affect of sex hormones on thymic vitality Over 100 years ago, gonadectomy in both male and female animals was shown to delay, but not prevent thymic involution. When done later in life, gonad removal induces a profound thymic rejuvenation. This has been done experimentally in animals and has been observed clinically in humans who underwent orchiectomy or oophorectomy for cancer. The molecular mechanisms of how castration regenerates the thymus are initiated in the “immature triple-negative compartment (CD3-, CD4-, CD8-) and early T lineage progenitors (ETP = Lin-, CD127-, CD25-. CD44+, CD117+). With aging, there is a reduction in the number of ETPs in the CD127- TN1 compartment. Castration restored the number of triple negative cells and the ETPs. The effects of castration in male mice are rapid. Within 6 hours, the levels of testosterone drop to 1% of normal levels. Within 10 days, there is a regeneration of the thymus, producing a cellularity that resembles a 2 month old mouse.
Pardoxically, gonadectomy temporarily increases thymic size, but then with time the thymus involutes again with aging. This is due to the decline in hGH that occurs with aging. Thus unless both the effects of sex steroids and the age-related decline in hGH are addressed, there will be a only a transient benefit to either gonadectomy or hGH administration in old age.
References:
- This publication goes back to 1924 THE INFLUENCE OF THYROIDECTOMY, GONADECTOMY, SUPRARENALECTOMY, AND SPLENECTOMY ON THE THYMUS GLAND OF RABBITS.
- Effects of Castration on Thymocyte Development in Two Different Models of Thymic Involution1
- Chemical gonad hormonal ablation. – Chemical ablation of sex steroids also induces a profound thymic rejuvenation. This is seen with the use of the drug that interferes with luteinizing hormone releasing hormone (LHRH, aka GnRH). Both LHRH antagonists and GnRH antagonists have been used to induce a chemical gonad hormonal ablation. Initially, only a GnRH agonist was available for prostate cancer (Luprolide, aka Lupron), but recently GnRH antagonists have also been developed. Paradoxically, however, GnRH agonists reduce IGF-1. This is a surprising finding, since IGF-1 actually induces thymic regrowth. Thus GnRH agonists may not help with thymic rejuvenation. (ref)(ref)(ref)(ref)(ref)
- Pregnancy – Pregnancy is associated with high levels of estrogen and progesterone. Accompanying these high levels of sex hormones, there is a dramatic thymic involution that occurs in the mother. In mouse models, the amount of thymic involution varies from 45% to 80%. Estrogen blocks early T cell development in the thymus. Estrogen induces a dramatic reduction in the thymic size and cellularity. All subsets of T cells decline (CD4 and CD8 cells). The triple negative cells (CD3-, CD4-, CD8-) did not progress from TN1 to TN2 developmental phases. Progesterone does not block T cell progression in the thymus, however. (ref)(ref)(ref)
- Adrenal corticosteroids – Suprarenalectomy (adrenalectomy) in rabbits was done 90 years ago and showed a dramatic effect in arresting thymic involution and results in dramatic thymic regeneration. Involution is permanently delayed, but in the rabbits there was regrowth of accessory adrenal gland tissue. Unfortunately, in the adrenalectomized rabbits, there was a very high mortality rate if future surgery on the rabbits was performed. This is thought to be due to the lack of stress hormones needed to cope with these major stressors, such as surgery. However, if gonadectomy and adrenalectomy were performed, permanent prevention of thymic involution occurred.
Chronic glucocorticoid administration has also been shown to induce thymic involution. This occurs because glucocorticoids induce apoptosis in double positive thymic T cells (CD4-, CD8- cells). The mechanism for this is that glucocorticoids like cortisol migrate into the cytoplasm of the cell where it binds to the glucocorticoid receptor (GR). This induces a nuclear translocation of the cortisol/GR complex where it binds to hormone response elements (HREs) in promoter regions of genes. This direct ligand/GR binding to HREs accounts for the positive control of gene expression (i.e. turning genes on) but does not account for the negative transcriptional control (turning genes off). Negative transcriptional control of gene expression by ligand/GR is mediated by interference with the cAMP-responsive transcription factor, CREB, and interference with transcription factor AP-1. In the case of NF-kB, cortisol/GR “turns off” NF-kb by inducing IkBalpha, a specific NF-kB inhibitor.(ref)
6. Zinc deficiency – Zinc deficiency plays a major role in thymic involution later in life (not puberty). Two distinct epithelial cell populations in the thymus produce a hormone called thymulin (aka Thymic Factor). It is a nonapeptide and requires Zinc for its biological activity. Thymulin is important in T-cell differentiation and with the enhancement of T cell and NK cell action. The lack of Zinc at the thymulin receptors on T-cells is a major cause of thymic involution. In studies done in mice, oral zinc supplementation in old mice for 1 month induced a complete recovery of crude zinc balance from negative to positive values. A full recovery of thymic function with regrowth of the thymus occurred with partial restoration of immune efficiency, as measured by NK activity. The 1995 publication Reversibility of the thymic involution and of age-related peripheral immune dysfunctions by zinc supplementation in old mice reported “A full recovery of thymic functions with a regrowth of the organ and a partial restoration of the peripheral immune efficiency, as measured by mitogen responsiveness (PHA and ConA) and natural killer cell (NK) activity, were observed after zinc supplementation. These findings clearly pin-point the relevance of zinc for immune efficiency and suggest that the age-related thymic involution and peripheral immunological dysfunctions are not intrinsic and irreversible events but are largely dependent on the altered zinc pool.”(ref)
- Magnesium deficiency – Mg++ deficiency has also been shown to accelerate thymic involution. This appears to be due to enhanced apoptosis and thymocyte sensitivity to oxidative stress. Under experimental conditions where magnesium deficiency is induced in rats, thymic involution started to occur within a few weeks after the establishment of magnesium deficiency. Up until recently, the molecular mechanism by which magnesium deficiency induced thymic involution was not clear. Today there is evidence that this may be mediated by inflammation, which induces an increase in IL-6. An increase in IL-6 occurs within 4 days following the establishment of Mg deficiency.(ref)(ref)(ref)
Clinical Significance of the Above
Q: Why does hormone replacement therapy with estrogen or testosterone in middle age cause thymic collapse?
A: When testosterone or estrogen therapy is given to men and women after age 50, there is an almost complete collapse of whatever thymus is left after the 30-40 years of thymic involution that has occurred since puberty. This is due to apoptosis of the thymic cortical thymocytes, primarily the immature, pristine clonal stem cells that produce the upstream triple negative thymocytes. Thymic expression of androgen receptors increases with age. This may be why the thymus involutes without any exogenous sex hormone replacement therapy. It is unclear if the thymic involution is due only to apoptosis of thymocytes or if it is also due to the apoptosis of thymic stromal cells. Most believe that it is both.(ref)
Q What might be done about thymic involution?
A: The simplest option would be to make sure that there is no deficiency in Zinc or Magnesium, since Zn++ is required for thymulin binding to the thymulin receptors. Magnesium should reduce IL-6 mediated thymic involution. Another more drastic option would be to undergo castration or oophorectomy, but this will only have a transient effect and may not be desirable for many obvious reasons!
Another option would be to administer Keratinocyte Growth Factor (KGF). KGF is already FDA-approved for oral mucositis prophylaxis in patients receiving myeloablative therapies before hematopoietic stem cell transplantation.
Another option would be to administer IL-7. IL-7 augments the survival of triple negative thymocytes and single positive thymocytes. Administration of IL-7 after periods of immunodeficiency enhances T-cell reconstitution. This occurs via increased thymic T cell development.
Another option discussed above would be to administer hGH. This has been shown to effectively regenerate the thymus in AIDS patients. However even hGH administration would not last if the issue of sex hormones was not addressed. For this reason another option would be to do chemical down-regulation of sex hormones, such as the use of Luprolide. Unfortunately, luprolide decreases IGF-1, so this may have an antagonistic effect on the treatment of thymic involution with hGH.
Final questions and answers
Getting back to the issue posed by the title of this blog,
Q Do I think it is feasible to develop an effective therapy for reversing age-related immune senescence, at least partially if not fully?
- Yes, with fairly high confidence. The required knowledge has been around for some time and the clinical trial for HIV patients establishes feasibility. The knowledge has simply not moved down the pipeline to where it is applied.
Q So, what has been stopping this from happening?
- One major barrier is that hGH and IL-7 are natural substances that can’t be patented. So, little money is to be made selling them and there is no incentive for a pharma or biotech company to get involved in a big way. Not so for recombinant versions, but these may be little better than the natural ones. Another barrier common to all therapies aimed squarely at aging is that you can’t conduct a FDA-approved clinical trial for a purely aging indicator. Finally, in the case of hGH, there is the possible issue of adjusting dosage so as to minimize possible pro-disease and pro-aging side effects.
- Then, will it happen?
- Yes, I think so as the market for scientifically-validated anti-aging interventions gathers steam.
MEDICAL DISCLAIMER
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION.


I’m surprised the role of melatonin was overlooked!
Thx Vince and Jim for a nice summation of the latest research on Thymic Rejuvenation.
I would like to direct your attention to the work of Vladimir Khavinson and other Russian researchers associated with the International Association of Gerontology and Geriatrics (Europe)
They show close immunoendocrine cooperation between the thymus and pineal gland.
Their research shows that “…involutive changes in the central organ of the immune system, the thymus, and the endocrine organ, the pineal gland, could be successfully restored with…peptides…”
http://link.springer.com/article/10.1134/S2079057011040096#page-1
http://eng.gerontology.ru/research/ageing/
There are very interesting papers on the rejuvenation of other organs like the pancreas and retia as well as effecting epigenetic (I think?) changes to DNA with peptides.
http://www.ncbi.nlm.nih.gov/pubmed?term=Khavinson%20VKh%5BAuthor%5D&cauthor=true&cauthor_uid=14501183
Another way to slow thymic involution may be to expose CMV etc. to the immune system by disrupting their lipid envelopes. This may lighten the burden on the immune system.
There is also some anecdotal evidence of the complete eradication of some infections!?
http://www.longecity.org/forum/topic/63422-antibiotics-could-cure-40-of-chronic-back-pain-patients/
http://www.longecity.org/forum/topic/42592-bht/
http://augmentinforce.50webs.com/BHT%20–THE%20HEALTH%20EFFECT.htm
Logic:
What an interesting trove of material,! Thanks. I will be digging into it and hope soon to be able to add to your comment.
Vince
It sounds to me that we may have a solution : progressive strength training fits the bill here very nicely. Growth hormone a by product of hard intelligent training of short duration. And at any age. Great article as usual!
Good point stevee222.
V.
Machine Ghost:
I think Melatonin does play a very important role in immunological processes, circadian regulation, and health maintenance, but not one central to the thrust of this article which is reversing adaptive immune system senescence. One of the many blog subjects sitting on back burners is a review of our hormonal system in which I will strive for a fresh look again at melatonin. We have a great schedule of topics lined up for the late Fall. Thanks for the prod.
Vince
Vince,
A major topic of your article is reversing thymic involution, and melatonin has been shown to do that, so I share Machine Ghost’s surprise.
“When testosterone or estrogen therapy is given to men and women after age 50, there is an almost complete collapse of whatever thymus is left after the 30-40 years of thymic involution that has occurred since puberty.”
The referenced article does not support such a strong statement. Nevertheless, if true, this would be an earth shattering discovery that would be picked by every media outlet to twist every bit of sensationalism out of it.
Since I am 61 and have been on an HRT for several years I am very interested in this topic.
Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression.
http://www.ncbi.nlm.nih.gov/pubmed/24905167