By Vince Giuliano
There are two distinct faces of life extension which are often confused, even by the best researchers working in the longevity field. This sometimes leads to paradoxical observations. I seek to clarify what these faces are in this blog entry, present evidence for the distinction between them, state how and why both are important, and relate them to longevity interventions previously discussed in this blog.

Two categories of processes and interventions exist that can lead to longer lifespans. They are very different. Image source
- Averting or neutralizing mortality factors, that is, actions and interventions which counter factors which are known to increase the probability of death.- leading to better health and longer functional lifespans. A great many such mortality factors are all-age killers. If you are shot, run over by a bus or fall off a cliff or incinerated by napalm, taking multiple dietary supplements or being of young epigenetic age won’t help you at all. There are thousands of such factors that can kill you rapidly or significantly increase the probability of your demise, including accidents, diseases, smoking, toxic environments, natural disasters and wars. For many species like wild mice – us no longer – predators who eat them are the main killers. All public health measures are intended to avert mortality factors – such as seat belt laws, clean air and water and good sanitization systems, and vaccination programs. These have been the main factors responsible for our long currently-expected lifespans. Finding better treatments for cancers and new cures for diseases are in this category, for example, In all of these cases there is an increased longevity of a population while epigenetic aging in that population is unaffected.
- Mortality can be induced by uncontrollable extrinsic factors which are independent of aging such as wars, road accidents, pandemics, natural disasters, or absence of medical health care. Because of such factors mortality of any population or individual can never be reduced to zero, even if actual age reversal is achieved.
Most “anti-aging” interventions’ are actually in this category, and do not actually affect aging. They increase expected lifespans, but do not impact your epigenetic age. Even some old standbys, like dietary calorie restriction, work to slow mortality at all ages and are in this category. “Dietary restriction (DR) is the most robust environmental manipulation known to increase active and healthy lifespan in many species. Despite differences in the protocols and the way DR is carried out in different organisms, conserved relationships are emerging among multiple species. Elegant studies from numerous model organisms are further defining the importance of various nutrient-signaling pathways including mTOR (mechanistic target of rapamycin), insulin/IGF-1-like signaling and sirtuins in mediating the effects of DR(ref),”
Some mortality factors are intrinsic, especially those which assume great importance in the advanced aged, including compromised vision and hearing, compromised kinesthetics, and the diseases associated with hyper constitutional inflammation -such as diabetes, dementias, arthritis, many cancers and gout. Many devices, work-arounds and treatments have been developed to neutralize these, examples being hearing aids, eyeglasses, cateract surgery, and prosthetic devices like canes, walkers,, wheelchairs, and grab bars. These all contribute to probable overall longevity, for some very little, but they are very numerous.
Interventions in this category are unlikely to affect our human lifespan limit of 122 years, no matter which of them, how many are simultaneously applied, or how consistently they are used. (A possible exception could be hypoxia-related interventions), Very few of us will live beyond 105 and we all will be dead before we reach 122 years – unless we can slow or reverse actual aging..
Slowing or reversing epigenetic aging. That is somehow slowing or turning back the ticking epigenetic clock in our DNA which is calendar-driven and which determines the multiple phenotypical symptom of aging on all levels – biochemical, cellular, tissue-specific, organ-specific, and systems specific. Specifically Steve Buss and I have seen aging being to a significant extent due to age-related histone double and triple hyper methylation at the H3k27me2-3 position, and consequent deactivation of multiple genes that govern natural repair, protective and restorative processes in the body. For example, tissue restoration due to stem cell differentiation is greatly reduced in the advanced aged due to such methylation,
Reversing biological epigenetic aging, what Steve Buss and I have called YOUNGING, is the only hope for anyone who wants to survive the vicious mortality probabilities that lead most of us to die by 105 and nobody to live beyond 122 years.
The case for aberrant histone methylation being the smoking gun that surely kills older people if nothing else is a strong one, Degenerative diseases and conditions of aging, the ones that surely kill older people, are only very rarely manifest in mice younger than a year, or humans younger than 30 years – when the lifelong accumulation of H3k27me2-3 histone methylation is still minimal and protective and restorative genes are still strongly active. ,(Of course there are numerous degenerative diseases of childhood and adolescence. The point is that they are not dominant killers as are the degenerative diseases of old age) A basic hope and intention of many longevity researchers is to prolong this protective state or to revert people to it – a process we have extensively discussed which we have called YOUNGING. See our Webcast Younging – Triggering Ancient Mechanisms for Rejuvenation, and our articles on YOUNGING in this blog written in 2022(res)(res)(ref)(ref)(ref).
These two kinds of life-extension processes have been confounded. Most of the putative “anti-aging interventions” pursued today are in the first category and in fact don’t impact actual or epigenetic aging. Interventions in this first category are good and useful. The traditional branch of medicine known as Geriatrics is concerned with many of them. Many other social innovations, like pushbutton activated pedestrian lights on busy streets are taken for granted. Several newer electronic techniques like video conferencing and GPS traffic guidance reduce time spent in traffic and traffic-related mortality. If we want long, productive happy lives we need to take advantage of them wherever they exist.
YOUNGING interventions that slow down or reverse aging are the long-sought Holy Grails of longevity researchers. We are well on the way to cracking that one, though we are not there yet. What is at stake is the possibility of lifespans of hundreds of years. We have learned much, including the root causes of aging and how in-principle to slow it down and reverse it. And recent experiments with small animals give powerful evidence for the existence and feasibility of age reversal. See the Section near the end of this document, SHORT UPDATE ON YOUNGING.
Yet, we still have no safe, practical interventions that can reliably induce systematic YOUNGING. One current barrier has to do with activation of the histone demethylase JDJM3, a necessary if not sufficient condition for YOUNGING. In short JMJD3 demethylation at H3k27me2-3 histone sites promotes he expression of multiple growth and development genes, which means it is implicated in the development of cancers. See, for example JMJD3 promotes survival of diffuse large B-cell lymphoma subtypes via distinct mechanisms. “JMJD3 stimulates the expression of proliferative-related genes and increases tumor cell growth, propagation, and migration in various cancers, including neural, prostate, ovary, skin, esophagus, leukemia, hepatic, head and neck, renal, lymphoma, and lung(ref).” In fact, a treatment strategy for some cancers like Lymphoma is inhibition of expression of JMJD3 via the recently developed drug GSK-J1. For discussions of both the benefits of JMJD3 and its roles in cancers, see the 2021 article The Functions of the Demethylase JMJD3 in Cancer. ..JMJD3, also known as KDM6B can be both an inhibitor and promoter of cancer “ Previous studies have pointed to a potential tumor-suppressive role of KDM6B in specific tissues (such as lung, colon, and pancreas) (30)(31)(32)63), while in others (such as blood, breast, and brain) it may play an opposite tumor-promoting function (24)(25)(26). This demethylase is an important player at the intersection between cellular senescence and cancer (22,29), and it also positively regulates epidermal differentiation (33). Interestingly, the KDM6B gene is located on chromosome 17 in close vicinity to the p53 tumor-suppressor gene, with allelic loss at this position occurring in a variety of cancers, including SCCs (1,22,(64)(65)(66)(ref)”.
WHAT IS AGING?
“Aging can be defined as the time-related deterioration of the physiological functions necessary for survival and fertility(Aging: The Biology of Senescence).” In humans aging appears to be a lifelong process manifest in multiple ways on all organismal levels. Aging involves shifts in biochemistry, the structures and operations of cells, organelles and organs, key processes like metabolism, organs, all body systems, and all key functionalities.”
The normal measure of aging of course is calendar age – how long a person has lived. Evolution has endowed us with pretty good natural capabilities for detecting and assessing advanced aging. Based on how a person looks, moves and talks we can generally estimate well whether that person is in his or her 60s, 70s,80s or 90 or beyond. Scientists have long sought a reliable biological measure of how long people have lived, looking for concentrations of substances in the body that correspond to calendar age. Starting with the work of Horvath at UCLA, it appears that DNA methylation best provides such a measure. Aging is accompanied by significant lifelong changes in promoter-site methylation of many key genes. For most genes, the state of promoter-site methylation changes year by year from birth to death. For some genes it systematically increases; for other genes it decreases. So it is possible to create “aging clocks” by systematically looking at methylation status of a few dozen or hundred genes see Jim Watson’s and my blog entry(ref):
“Why is DNA methylation important?
This blog entry looks at aging, health and disease from the viewpoint of DNA methylation, that is, lifelong changes in methylation status of selected genes. This is a highly useful viewpoint because it:
- provides a theory of aging and a fairly concise definition of aging,
- provides the most concise measures of human aging we have, and
- is a good predictor of all-cause mortality.
According to this viewpoint, genomic methylation:
- plays crucial lifelong roles in human development and maturation, from embryogenesis right up to death,
- is one of the three major epigenetic mechanisms for gene activation and silencing,
- is a major causal factor in the program of aging,
- provides an explanation of how chronic inflammation accelerates the aging program,
- explains several other basic known mechanisms and effects of aging; E.g. loss of border proteins that protect CpG islands from methylation, and the fact that men age faster than women, and why women’s breasts age faster than the rest of their bodies,
- provides insights into the programs of aging that suggest possible hacks on those programs,
- has molecular mechanisms which are fairly well understood and well documented,
- helps explain a number of disease processes and disease susceptibilities. how hypermethyltion of the BRCA1 gene confers susceptibility to breast cancer,
- helps to explain how chronic stress leads to accelerated aging,
- plays a direct role in the pathogenesis of diabetes, cancer, other diseases,
- with aging, increases expression of repetitive DNA sequences and human endogenous retroviruses so as to lead to chromosomal/genomic instability, telomere attrition, and aneuploidy,
- helps explain selective hereditability of traits, and
- reveals important links to a number of other important topics and viewpoints related to health and aging we have discussed over the years such as other aspects of epigenetics, events in the NAD world, stress and hormesis, oxidative stress, importance of circadian rhythms, transposable elements and alternative splicing.”
PURPOSE OF AGING
Surprisingly, there is lack of agreement among scientists about the purpose or nature of aging, A traditional view is expressed in this quote: “Many evolutionary biologists (Medawar 1952; Kirkwood 1977) would deny that aging is part of the genetic repertoire of an animal. Rather, they would consider aging to be the default state occurring after the animal has fulfilled the requirements of natural selection. After its offspring are born and raised, the animal can die. Indeed, in many organisms, from moths to salmon, this is exactly what happens. As soon as the eggs are fertilized and laid, the adults die. However, recent studies have indicated that there are genetic components to senescence, and that the genetically determined life span characteristic of a species can be modulated by altering genes or diet(ref).” This traditional view is no longer held by most longevity researchers.
Aubrey de Grey, a prominent aging researcher, and his followers continue to think that aging is caused by accumulated damage due to wear and tear(ref). This approach to longevity is an engineering repair-shop one. “If something in the body breaks with aging, fix it.”
However, most longevity scientists today, including myself, think that aging is not in fact genetically encoded or due to wear and tear. Rather, it is epigenetic, due to systematic lifelong changes in our DNA, particularly methylation status of numerous genes and changes in chromatin structure. With the genes themselves remaining the same. With advanced aging, gene regulation is so dysregulated that they can no longer do their jobs. Evidence for this view is that with advanced aging, hundreds or thousands of genes concerned with natural restorative and repair on multiple levels in our bodies become gradually repressed due to age-related excess histone methylation at the H3K27 locus, and can no longer keep us young and healthy. I have discussed this situation and its implications in several blog entries this year(see entries in this list).
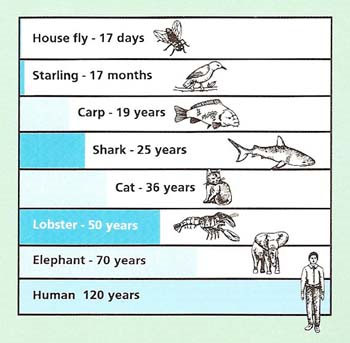
Approximate maximum lifespans Image source
Members of virtually all species, us humans included, age, although at radically different species-determined rates, and are subject to species -determined maximum lifespan. Even if we live to be in our 90s or 100s, we humans can expect with absolute historical certainty to die of one cause or the other by 123.
A typical pattern for mammals, including humans is a period of adulthood (up to around 27) where all-cause-mortality (ACM) is relatively low and fairly constant, followed by a period where numerous mortality factors become increasingly important (up to around 70), followed by old age where mortality factors steeply increase in importance. All-cause mortality (ACM ) then continues to shoot up radically, becoming so large humans are near-certain to die before age 115.
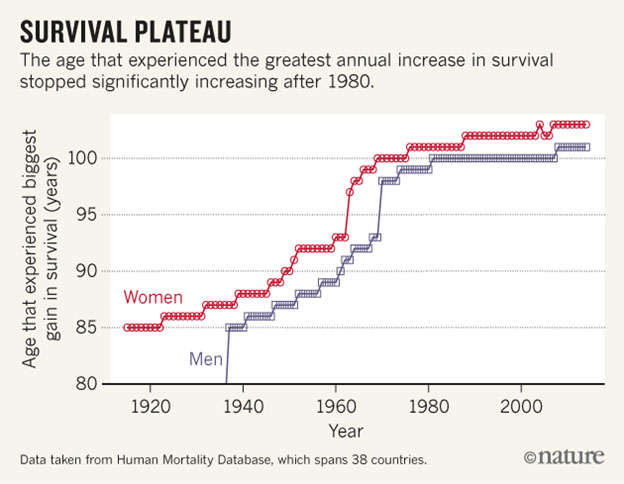
Image source Human lifespan appears to max out at 122, We don’t know for sure that scientific advances will allow us to increase that number significantly.
It is controversial, as to whether maximum human lifespans may shift as time goes on (ref). Certainly, expected lifespans of human populations have increased dramatically since pre-historical times, though they are affected by matters such as the COVID pandemic “They estimate that in the U.S., life expectancy — how long a person born today is projected to live, based on current death rates — has now dropped by almost two years. The U.S. declared a public health emergency related to the coronavirus in February 2020. Prior to that month, the nation’s life expectancy was 78.8 years(ref written April 2021).” Some think we humans have already reached our maximum lifespan of 122 (ref). Others think that expected and maximum human lifespans are correlated and may be expected to increase indefinitely as the years roll on(ref). As I see it, every species has its own version of a program of aging, and that program fixes maximum lifespan for members of that species. If aging were a matter of random damage, we would see a very few 10 year old mice, a very few dogs who live to 100, and a very few people who live to 300. We do not.
My opinion is that the human lifespan limit of 122 will soon be significantly transcended and some us will be able to live hundreds of years. My optimism is based on the fact that several groups are now researching age reversal approaches using small animals. Descriptions of progress are appearing every few week. My guess is that effective age reversal will clearly be demonstrated for a tiny number of people within 3-5 years from now. My intent is to be part of that group. However, it may require 15-25 years for YOUNGING to be practically available to the general public.
PHENOTYPES OF AGING
Phenotypes of aging are typical observed consequences – hundreds of them, for humans at all levels – biochemical, cellular, cell components, organs and organelles, and major body systems Some of these provide the clues we use to deter advanced aging, like skin wrinkles, grey hair or no hair. Many of these can be causative of diseases, like body states of hyper inflammation, or vulnerability for several diseases like cancers, Diabetes and Alzheimer s Disease.
Some phenotypes of aging have over the years been thought to define aging or be causes of it. I think this is a mistake. An example is telomere shortening. 14 years ago when I seriously started my studies of longevity science, like several scientists at that time I thought:
- In general, with advanced aging telomeres (specialized protein caps at the ends of chromosomes) tend to become shorter,
- A consequence of too-short telomeres is genomic instability, implying dysregulation of protein expression, causing what we experience as aging, and.
- Therefore a powerful longevity-promoting therapy would be stimulation of production of telomerase, a natural enzyme our bodies use to extend short telomeres
This line of argument for a root cause of aging appeared compelling but turned out to be contradicted by several facts, among which are 1 telomere lengths in humans wax and wane due to a variety of factors and are naturally coregulated by several feedback loops, 2, When telomeres get to short, our bodies at all ages tend to upgrade the expression of telomerase and make them longer again, and 3. Promotion of telomerase, although proposed as a life-extending strategy more than 17 years ago, has never been proven to be that. So shorter telomeres with advanced aging is one of hundreds of phenotypes of advanced aging but neither defines nor causes advanced aging,
Many phenotypes of advanced aging are in themselves mortality factors which move to center stage typically around age 75. Loss of good kinesthetic balance is an example, which can result in bone-shattering mortality-inducing falls. Another is the induction of chronic inflammatory state which can contribute to many pathologies of old age including to auto-immune diseases, diabetes, and many cancers. Another is growing vulnerability to dementias like Alzheimer’s Disease.
ADVANTAGES AND DYSADVANTAGES OFINTERVENTIONS THAT INCREASE LIFESPAN
As pointed out above, we can distinguish two categories in which these belong:
- Interventional that slow, mitigate ot prevent operations of mortality-inducing factors
Advantages of these include:
- There are a great many of such interventions.
- Many of these are applied socially in our society , such as sanitation and safety laws, clean air and clean water regulations.
- Many others are readily available and economical for individuals to practice.
- How they work is usually readily understandable.
- Results of their application is cumulative up to a point.
- Living according to a properly selected regimen of them can probably enable most of us living in advanced societies to be highly functional and live full lives until age 95 or beyond (not applicable for a limited set of us who have identifiable genetic disease predispositions).
- They are likely to be necessary even if advanced interventions are discovered and implemented that actually address aging.
- You can’t live to be a healthy 200 or 300 unless you live to be healthy and functional100 first. I think we now know how to likely do that. And I am doing it personally.
- These interventions got me to where I am now.
Disadvantages of these
- They are unlikely to get most of us in a highly functional state much beyond age 100.
- They are highly unlikely by themselves to extend the maximum observed human lifespan beyond 122 years.
- To the extent that deaths are caused by uncontrollable events like natural disasters, plagues, wars or shootings, even those following he most rigorous life-extending program cannot be guaranteed very long lifespans.
Interventions that actually slow or reverse epigenetic aging
Advantages of these include:
If and as these become available they could enable maximum lifespans of hundreds of years or more, since they address the actual issue of aging
Disadvantages of these
- Basically, none exist for humans now, though there is much promising research that some may be known soon.
- A clinical trial that definitely establishes the efficacy of an intervention without side effects would have to run 30 years or more.
INTERVENTIONS DICUSSED IN THIS BLOG ARE OF WHAT KINDS?
Essentially all interventions discussed in the history of this blog except the ones related to YOUNGING are ones that slow, mitigate or prevent operations of mortality-inducing phenotypes of aging that are themselves mortality factors:
- Dietary restriction-promoting techniques
- Drugs known to be life-extending such as metformin and rapamycin
- Techniques that depend on Hormesis, be these exercise, or heat-shock or old-shock approaches
- Good social interactions
- 40 haze vagal system stimulation
- Regular patterns of sleep maintaining
- Oxygen therapy
- Reduction of chronic inflammation
- Sanitary conditions; clean air and water
- Dietary supplements in general
Many interventions in the first category are highly likely to be necessary for YOUNGING although by themselves not sufficient for YOUNGING to occur. These include:
Reduction of chronic inflammation, this being necessary for expression of JMJD3, the demethylase necessary for activation of multiple restorative and repair genes associated with the H3K27me2-3 histone site. We have hypothesized that such demethylation is a necessary condition for the process we have identified as YOUNGING01(ref).
Good regular sleep
Regular physical exercise
Alternative H3k27me2-3 histone demethylases
In earlier blogs on YOUNGING01 as well as this one, I have emphasized the importance of histone demethylation at the H3k27me2-3 position as a means of activating large numbers of genes involved in natural restorative process. I have focused almost exclusively on the JMJD3 demethylase – also known as KDKM6B However as pointed out above, JMJD3 activation can be involved un and trigger certain cancer processes, so great care must be taken in considering its use as a longevity anti-aging treatment. Actually the situation is more complex where three different histone demethylases work at the H3k27me2-3 position, and safer initiation of YOUNGING01 may entail activation of more than one of them. These three demethylases are
- JMJD3 also known as KDKM6B
- UTX also known as KDKM6A
- UTY, a relatively feeble demethylase not discussed much in the literature
The roles of these demethylases in developmental and cancer processes are treated in the 2019 publication The histone demethylase UTX/KDM6A in cancer: Progress and puzzles.
SHORT UPDATE ON YOUNGING
In the last ten days, the popular news media including CNN, TIME magazine, and the New York Times have carried stories about Dr. David Sinclair’s work on age reversal and how YOUNGING in laboratory mice was actually demonstrated in his lab. David, of course, is a Professor at the Harvard Medical School, and is responsible for a major Lab there that has conducted several important research studies related to aging. He has gained considerable notoriety for his various works. He is author of the book Lifespan: Why We Age and Why We Don’t Have To. David has used three of the four Yamanaka factors (Oct3/4, Sox2, Klf4,) delivered by viral vectors to reverse epigenetic again in mouse systems, back to the adult stage when reproduction is finished. David promotes a theory of aging – loss of epigenetic information – which I believe is highly credible. . David does not use the term YOUNGING as Steve Buss and I have and only mentions but does not highlight the importance of histone demethylation, as we have. But David highlights the possibility of systematic age reversal as we do. The CNN link contains a video of David talking this work. Two of the recent publications coming out of his lab that describe recent work in detail are: Loss of Epigenetic information as a cause of mammalian aging (published 8 days ago) and a paper on mouse vision restoration Reprogramming to recover youthful epigenetic information and restore vision (Dec 2020)
COMING SOON IN THIS BLOG
I celebrated my 93rd birthday, on November 17, 2022. I will discuss my continuing personal heath, vitality, and productivity and how I keep these up in a soon-forthcoming blog entry. I will broadly characterize the lifestyle actions and interventions that I believe have kept me largely heathy, active. functional and productive up to this point. And, I expect, at least until I am 100. Finally, I will describe a new educational program I am now planning, the intention of which is to empower a small number of others in their 60s and 70s to have another 20 to 35 years of health, vitality and full functionality as I have been enjoying.
For those of you not satisfied with the 20-30 years of life extension that can be offered to you right now and want more, I have the following reminder to offer. If you want to live until you are 300 in good shape, you have to make it to 100 first in good shape. I believe I can advise you on steps that will provide you a good probability for doing that. Hopefully, by the time you get to 100, science will provide you and me with means to reach 150 in good shape. That is what I am personally betting on.


Happy belated birthday Vince, if it were possible could you please update your current supplementation regiment, please