By Vince Giuliano and Steve Buss
(A general introduction to this first substantive Blog Post in a new YOUNGING Series is provided in the post Introduction to the YOUNGING Series – Emerging Aging Reversal Strategies and Treatments). After reading the present basic post you might want to go on to read YGC MORE ON YOUNGING1.0 – THE EMERGING AGING REVERSAL STRATEGY and YGD YOUNGING1.0 PART 4: UNDERLYING MECHANISMS OF YOUNGING 1.0. HSC STEM CELL DIFFERENTIATION.
The key elements of a strategy for age reversal are emerging in the recent literature –we call it YOUNGING1.0. This blog entry describes our understanding of that strategy, its elements, how it works and its results, and cites research evidence for its salient aspects. This is possibly the most important blog entry published over the 12-year history of ANTI-AGINGFIREWALLS. That is because such a strategy – where human physiological age is actually and significantly reversed –once demonstrated will be unique in our history of all humanity. Age reversal has happened in stories and myths, such as the story of Dr. Faustus and vampire myths, and the power of Fountains of Youths, a number of which can be visited in the world today. Vince first visited the Fountain of Youth Archeological park in Saint Augustine Florida when he was only 4, and has since visited others including the Fontana di Trevi in Italy, the Baños de Coamo in Puerto Rico and. Tambomachay in Peru.


But though much sought after, true YOUNGING approaches have never existed as the result of an actual scientific intervention. We seek here to lay out the elements of a science-based age reversal strategy, one that works in small animals which we believe will work in humans as well, and one that is actionable now. We draw those elements from studies by numerous specialized researchers, many of whom are unfamiliar with the work of the others. So, we are seeking to fit together pieces of knowledge from separate sources to constitute a whole new framework. This characterization of YOUNGING is as complete as we can make it now, and we believe it to be compelling. True, major gaps in our knowledge are still to be filled in. We proceed first in this Section I by listing key assertions without digressing into the backup science and literature, and in Section II back up our key points with additional information, research literature sources and citations, and identify some important still-unanswered questions.
SECTION I KEY POINTS
- YOUNGING is real. It has been demonstrated in small animals and by much research which characterize and validate its details.
- The evidence is strong that YOUNGING will work in humans. It has been demonstrated to work in rats(ref). Of course, all statements we make here with respect to human YOUNGING are conjectural until it is actually demonstrated by well designed clinical studies. However, the genetic and epigenetic compositions of humans and rats and associated pathways and process in humans (and in fact of all mammals) are so evolutionarily conserved and nearly identical, that we assign a high degree of certainty to the basic points made here. The research literature citations in Section II associated with each of these points convey why we are so certain.
- YOUNGING is the result of an epigenetic program. In this respect it is a program like aging itself, running the aging clock backwards. You can think of it as a program or subroutine that replaces or runs simultaneously with the aging program itself. We have good ideas about the nature of one such program we call YOUNGING1.0, how to trigger its activation, how it works, how long it runs, and what its biological youth-generating results can be.
- YOUNGING1.0 as described here is a long process lasting years, not a one time event. It is not something that happens right away or overnight. In this respect it is like aging itself, a process that goes on in our biological backgrounds that is not naturally perceptible by us moment-to-moment, or even day-by-day or month-by-month. Rescaling of rat results suggests that YOUNGING1.0 in humans over 40 may take 10 or more years to fully play out. This means we won’t see convincing clinical results for humans that YOUNGING exists and works until 8 or more years after a valid-scale clinical study is set up. Until then many people may choose to deny its reality, and others like us will have to depend on the indirect but yet-powerful evidence and powerful highly personal experience.
- There are other epigenetic programs that foster YOUNGING besides that described here called YOUNGING1.0. We mention a few of these here.
- YOUNGING1.0 is a process that can be initiated by disease-free adults, intended to bring them back towards or to an epigenetic aging state of a typical 20 year old. It is known that there is a major shift in epigenetic gene regulation in humans typically centered around age 24, where numerous growth and protective genes are down-regulated and inflammatory and pro-aging genes are upregulated. It is interesting that this timing coincides with the end of the main child-bearing years for humans as we used to be long ago. So the shift from self-protection may have had an evolutionary purpose. The major driver of this shift is relatively sudden methylation and consequent silencing of numerous growth, development, and protective genes.
- Initiation of adult aging around 24 years of age appears to be an evolutionarily conserved process triggered by histone methylation by the double and triple histone methylases H3K27me2/3. That is, di- and trimethyl-lysine 27 on histone H3. This results in silencing of numerous genes, including many anti-inflammatory and anti-aging genes that are largely active in youth. The results of this methylation is down-regulation or inactivation of protective genes related to this particular histone position. These include genes that encode for protective heat shock proteins. This may be the essence of adult aging as we know it. This evolutionarily-conserved shift has been well-studied in nematodes where it all happens in only 4 hours. This same histone methylation process also happens in plants as they shift from their juvenile to adult phases. The YOUNGING1.0 process seeks to reverse this genome-wide methylation and restore the protective gene activation patterns of a much younger person, at an extreme for humans. that of a typical 20 year-old.
- H3K27me2/3 and its methylation/demethylation roles in growth and development are important and manifested in plants as well as animals. So, these matters appear to be very ancient and core to life cycles of most if not all living things,
- YOUNGING1.0 is a complex process that proceeds in well-defined stages. In outline:
- Initiation by introducing exogenous substances and/or processes that promote active genome-wide demethylation of histone proteins H3K27me2/3. I believe this can be accomplished many ways, rapidly or slowly.
- Rapid body response by downregulation of inflammatory cytokines IL-6, TNFalpha and IL1alpha. Rapid response, in a few days. This has been observed in rats, we believe as part of YOUNGING1.0. A great many or most serious longevity researchers agree with me (Vince) that drastic reduction of systemic inflammation is absolutely necessary for any form of cell or organ-level renewal or regeneration to take place. It is not known however whether bringing systemic inflammation way down to 20 year-old levels and holding it down is sufficient by itself to initiating and maintaining a form of Nor do we know for sure that this process will be free of negative side effects in humans.
- Global responses in re-modeling of gene regulation, including upgrading DNA repair and many other “health and longevity” genes like HO1 and NRF2 and downregulating of-inflammatory and aging” genes, like IGF-1 and MTOR. Time frames unknown but probably fairly quickly. These first 3 steps set up and launch the program, but they are not yet the main program itself.
- Epigenetic, biological pathway, organ, and systems remodeling, including changes in DNA promoter methylation. Inducing such gradual changes is the main operation of the YOUNGING. In human years the process is estimated to go on for 8 or more years.
I expand on these points below.
- It is very difficult to know if YOUNGING1.0 is going on in someone, except after the process has gone on for four or so years. After that time DNA methylation testing should show the individual as significantly younger than expected for his or her age cohort and/or younger than before initiating the YOUNGING1.0 DNA age testing will not work as a measuring tool before that time lapse because it is predictive of biological age only with a 3 year error spread. One could ask “YOUNGING1.0 is initiated by H3K27me2/3 de-methylation which happens right away, so why would that change not show up in a DNA methylation test?” The answer is that DNA methylation age tests look at something else. They look at the methylation states of the promoter regions of a hundred or several hundred key genes , whereas H3K27me2/3 applies to only specific histone site (lysine 27 of histone 3). There is such a histone site on every nucleosome and an estimate of over 100,000 nucleosomes for every chromasome, and 23 chromasomes in humans. So it is clear that methylation or demethylation of the single histone site H3K27 may affect the methylation status of and therefore the activation status of hundreds of thousands of genes. DNA methylation tests will only show a person as younger after the process has been running for several years. [I (Vince) experienced that back in 2017 when I did DNA aging tests just before and just after infusions of plasma derived from very young blood. Within expected margins of error, the tests, showed no differences in my DNA-predicted age. At the time I thought that result meant the infusions did nothing, because I did not comprehend the long process nature of YOUNGING. Only now in late 2020 do I understand that with those infusions a YOUNGING1.0 process may indeed have been kicked off in me, a process that is still running today and may go on for 8-10 years more. I will soon do another DNA methylation test to check that hypothesis out.]
- There are many seemingly practical approaches to demethylating H3K27me2/3 and thus to initiate the YOUNGING. The main substance that specifically demethylates H3K27me2/3 is JMJD3. The Jumonji domain-containing protein D3 (JMJD3), specifically demethylates di- and trimethyl-lysine 27 on histone H3 (H3K27me2/3). So we can ask “what substances can we practically use to activate JMJD3 and what other substances cab be used to demethylate H3K27me2/3? It turns out the list of familiar substances that can do that is quite long, including:
- Certain familiar herbal substances, including Curcumin, and High AkBA Boswellia
- DHEA
- Alpha keto-gluterate
- Vitamin D
- and dozens of others to various extents
- And also processes such as fasting and breathing supplemental oxygen
- These approaches may vary in their efficacy, speed of activation initiation , side effects, and safety. We do not know which ones will really work or are best for humans right now. The desired effect is demethylation of H3K27me2/3 and this is likely to be possible via other demethylases besides JMJD3 and other demethylating processes..
- One approach to initiating the YOUNGING1.0 process, used for animal experiments by Harold Katcher and his colleagues used a substance they call Elixer as an activator.
- Elixer is administered in rats as a series of two IV administrations a week apart. This is all that is required to initiate the YOUNGING of rats by multiple months equivalent to 8-12 years of human life.
- These experiments are critical elements of the proof of the YOUNGING However the entire YOUNGING1.0 concept as outlined here is was put together by us, Vince and Steve. Katcher’s recent paper, co-authored by Steve Horvath and many others, covers the results of the YOUNGING of rats, but does not characterize the YOUNGING-process, or the roles of H3K27me2/3 or JMJD3 as covered here, or how it is initiated. We, Vince and Steve, are responsible for what is in this blog entry and Harold Katcher and his colleagues might take issue with some of the things we say here.
- Pending issue of a patent, Katcher and his colleagues are keeping the content of Elixer as a trade secret. They have gone so far as to say, however, that it contains only substances that are found in the blood of younger animals
- Because of the importance of Dr. Katcher’s work and what he and his colleagues have already published, this Anti-agingfirewalls blog is featuring a series of video interviews that we (Vince and Steve) have had with him which we expect to publish very soon. Steve Buss is the Producer of the series and Vince is actively participating as Executive Producer.
- A second approach to initiating YOUNGING, is the infusion of plasma derived from very young blood, picking up from the long-known results of hetrochronic parabiosis experiments. When old and young mice or rats are sewed together so that they share a common bloodstream, the old mice/rats get demonstrably younger and the young mice/rats get demonstrably older. The conclusion is that there must be factors in the blood of young animals that can rejuvenate older animals.). In my case the infused plasma was derived from blood in discarded umbilical cords of newborn human babies. Use of plasma (which is the blood fraction left over when blood cells are removed) instead of whole blood avoids issues of blood type and graft-host rejection.
- A third quite different approach to initiating YOUNGING1.0 than an IV one could be to use enhanced oral delivery approaches for JMJD3 activators or combinations of JMJD3 and other HK27me demethylases. This is a speculation on our part and we have questions on the safety of activating JMJD3 which are discussed later in this blog. These are approaches that are designed to provide significantly enhanced bioavailability than that of normal pill, powder of liquid delivery. In particular, we have been considering liposomal delivery, an approach where active substance ingredients are encapsulated in nano-sized lipid (fat based) particles. The result is greatly enhanced freedom of the carrier particles to travel through the body and get where they are needed. Liposomal delivery technology was pioneered by the pharma industry and a number of liposomal drugs as well as liposomal dietary supplements are now being marketed..
- (Vince personal comment) It turns out I have also been pursuing that third approach for a number of years now, without knowing the YOUNGING1.0 reason for doing so or ever having heard anything about JMJD3 or H3K27me2/3. Specifically, going back 5 years ago I (Vince) developed and started to consume a liposomal preparation of concentrated extracts of curcumin, high-AKBA Boswellia, ashwagandha and and ginger. I did this to control chronic inflammation, because I knew these four herbal ingredients were powerful suppressors of systemic inflammation, and because I knew that bioavailability of these substances was normally very limited. See the blog entries The Making of a Dietary Supplement and INFLAMMATION PART 6:THE SCIENCE BEHIND THE 4 HERB SYNERGY DIETARY SUPPLEMENT.
(Disclosure: . Regular readers of this blog know that because this product has worked so well in controlling chronic inflammation for me, and for members if my family and friends, we have created this liposomal concoction as a commercial product, called 4 Herb Synergy. Vince’s family supplement company SYNERGY BIOHERBALS has been marketing that supplement for over a year now.)
What I did not know at the time I wrote these articles is that curcumin and high-AKBA Boswellia are in fact JMJD3 activators and therefore H3K27me2/3 demethylators. I strongly suspect the ginger and ashwagandha exercise similar impact Since I have been taking either the home-made or the commercial 4 Herb Synergy product daily for over 5 years now, and because I also take substantial daily doses of DHEA and Vitamin D, it is likely I have been in fact to some extent been activating JMJD3 on a dialy basis.
- (Vince comment continued) I don’t know the extent to which the blood plasma infusions or consuming the liposomal substances, DHEA and other JMJD3 activators has activated YOUNGING1.0 in my body. I have a number of clues however, which suggest that the answer may well be yes, The first observable effect, drastic reduction in expression of inflammatory cytokines and inflammatory indexes was achieved years ago. And I am free of debilitating age-related disease conditions that haunt people in my age range (now approaching 91) and I seem to be as active and productive as ever. My medical blood tests, vision and other tests seem to show that most systems are stable. Some measures like my lipid scores are greatly improved over what they were years ago. Biomarkers such as resting heart rate and HRV as measured daily by my OURA ring are usually very good to excellent. I can walk 2-3 miles on rough and hilly terrain in the woods by my house without problems. My reading, research and thinking and writing seems as good as ever. Have I in fact been YOUNGING? I don’t know for sure but will soon be pursuing a new round of DNA methylation testing to help find out.
- Breathing oxygen in combination with exercising may promote YOUNGERING1.0. Oxygen is one of the promoters of JMJD3. There is an older but significant body of science behind achieving healthful results via interventions that involve routine breathing of oxygen while exercising. Devices can be purchased that make it practical and easy to do so at home. Steve Buss has been experiencing the benefits of this intervention for years now. He intends to cover this subject in detail in a follow-up blog entry. That blog entry will cover the science and historical background of the exercise-oxygen approach, recent related research, how the practice works in detail, the equipment required and where to get it, and the benefits of the approach that Steve has personally experienced.
- The biggest danger for YOUNGING1.0 or any other age-reversal approach is the initiation of cancers or acceleration of growth of unknown cancers already in a person. This is because many of the genes upregulated in any cell or organ renewal approach are also the same ones commonly characterized as oncogenic (cancer-creating) genes, such as the Yamanaka stem cell renewal factors, OCT-4, SOX2, KLF4 and C-MYC – or OSKM in short for all of them.
- Demethylation of H3K27me2/3 in cancer cells is not a good idea because it can lead to cancer proliferation. The advantage of the dietary supplement approach to JMJD3 activation Vince has been pursuing as outlined above is that each of the 4 herbal ingredients in 4 Herb Synergy is also an inhibitor of cancers. Each powerfully acts to inhibit NF-kB, the key protein required to activate systemic inflammation, maintain inflammasomes and allow cancer progression. Cancer processes are inherently inflammatory.
- There are other known natural programs that run in the bodies of normal people that can contribute to YOUNGING. For example, senescent cells exude the inflammatory cytokine IL-6. IL-6 exuded by senescent cells is a necessary trigger for nearby normal cells in the same organ to start expressing the OSKM Yamanaka factors so they can epigenetically regress to becoming stem-cell like progenitor cells in the same lineage type, which can the differentiate to replace the senescent cells
- The neighboring cells in a single organ may be in quite different epigenetic states than those in neighboring cells. Some may be responding to a normal aging program. Others may be senescent and responding to a program for senescent cells, other cells may be engaged in a YOUNGING We think what happens to the organ depends on the relative balances of cells in these different programmed process, and perhaps on a variety of additional signaling factors as well. Recent animal experience suggests that for normal people and most organ systems, the numbers of cells in YOUNGING decline and the numbers of cells in the senescent state increases as people move beyond middle-age. So organs age and eventually tends towards dysfunctionality or susceptibility to disease. On the other hand a simplistic viewpoint is that if enough of the cells in an organ are engaged in YOUNGING, the organ itself may YOUNGER. Harold Katcher has made a strong point that the age of a cell depends not on its history but on organ and body environmental factors which drive that age. We think he is right about this.
- In addition to the approaches mentioned for initializing YOUNGING1.0, there are doubtlessly a continuum of others involving different stresses or activator of JMJD3 or other histone demethylases administered via different channels and in different doses. It may take years to sort out which ones are safest and work best. In particular the dosage effects may be highly nonlinear, where too-little could have only negligible effect and too much unsafe.
- An entirely different approach to YOUNGING has been demonstrated to work in old mice, which is simply to drain off half the blood plasma and replace it with a solution that contains albumin. A twist on the old and long discredited medical practice of bloodletting. It seems that what helps here is removal of pro-aging factors from the plasma in the bloodstreams of older animals.
- What is known about YOUNGING and YOUNGING1.0 leaves many open questions. For example, as already pointed out, questions include:
- What is the best way to initiate YOUNGING1.0?
- How can I know if YOUNGING1 is going on and the extent to which it is going on without waiting for years to see?
- Can YOUNGING1 have undesirable side effects irrelevant for rats but important for us humans? (Vince): I am happy to experience considerible epigenetic age regression, and would love to regain some things I had earlier like thick black hair on my head and more acute hearing. But I don’t want to lose any of my memories or give up any of the wisdom I have gained through aging. I don’t want any basic personality changes to occur or any loss of lovingness and affection. I would like some 45 years of epigenetic regression, but don’t want to lose what I have learned about longevity science in just the last dozen years, not to mention last week.
- Can the YOUNGING1 program run too long or go too far once it is started?. Can it easily be turned off?. (Vince): I don’t care to be a bumbling teenager or adolescent again. I would like some restoration of my sex drive, but I don’t want it running me like it did when I was 22.
- What about some of the negative consequences associated with JMJD3?. Recent literature suggests that inhibition rather than activation of JMJD3 may be helpful for treating certain conditions such as osteo-arthritis and certain auto-immune conditions. JDJM3 promotes the differentiation of stem cells into various somatic cells. By the same token it tends to slow down or interfere with cell de-diferentiation via the Yamanaka factors, and epigenetic regression of somatic cells into stem cells, an important natural renewal process
- YOUNGING approaches will in all likelihood allow many existing humans to live for many more chronological years than 123, the maximum chronological age any human being in history has been known to live. Not by strengthening us somehow so we can bulldoze through that limit, but by allowing us to get nowhere near the internal biological stress conditions that normally stop human life at or before that age.
- This of course is a conjecture, and a hope many of us have to be one of those humans.
- This may require triggering multiple bouts of YOUNGING while the chronological years roll on.
- I conjecture that a few other YOUNGING approaches in addition to YOUNGING1.0 will be required to get past 123 This is because YOUNGING programs appear to vary in their efficacy according to organs and body systems
- (Vince): I comment personally that until just now, I could not imagine any way for members of our species to live beyond the 123 chronical years limit. There is a completely documented maximum chronological age for members of every known biological species, ranging from minutes or hours for some insects to thousands of years for certain tree species. I could not imagine that we humans could find an exemption to that rule. Now I can. So I see this possible avenue for breaking through the chronological age limit to be very exciting.
- A number of other interventions have been showed to extend mammalian lifespans somewhat, but our sense is that they fall short of initiating a full YOUNGING process such as that described here. These include using nicotinamide riboside to upgrade NAD+ expression, pulsed taking of rapamycin, and taking of metformin. Some of these are interesting from the viewpoint of YOUNGING because of their mechanisms of operation. For example, nicotinamide riboside upgrades the mitochondrial unfolded protein response (UPR), a process we think take place in YOUNGING1.0
SECTION II: SCIENCE AND LITERATURE BACKING UP KEY POINTS
- YOUNGING is real
Independently of the details presented here, a surprising number of researchers have come to this conclusion based on earlier evidence. See these publications for example:
- Therapeutic potential of systemic brain rejuvenation strategies for neurodegenerative disease. 2017
- Mechanisms of Hippocampal Aging and the Potential for Rejuvenation. Epub 2017
- Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. . Epub 2014
- Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. 2014
- Aging and brain rejuvenation as systemic events 2015
The Katcher-Horvath July 2020 publication Reversing age: dual species measurement of epigenetic age with a single clock
This publication is very important because it offers evidence that makes a number of the points cited here very clear. The paper has many authors but two of particular importance are Harold Katcher who responsible for creating the YOUNGING intervention and the experiment administrating it to rats, and Steve Horvath who was responsible for creating the specialized DNA age testing protocols involved. The paper is well-worth careful reading if you have not already done so.
This paper is about research with older rats who were infused with an unknown proprietary substance developed by Dr. Katcher called ELIXER, presumably related to youth-inducing factors in the blood of younger rats. The paper describes how Dr. Horvath developed six tissue-specific epigenetic DNA methylation clocks. Two of these clocks that can be applied for both rats and humans because of the near-identical genes of both species. “Young blood plasma is known to confer beneficial effects on various organs in mice. However, it was not known whether young plasma rejuvenates cells and tissues at the epigenetic level; whether it alters the epigenetic clock, which is a highly-accurate molecular biomarker of aging. To address this question, we developed and validated six different epigenetic clocks for rat tissues that are based on DNA methylation values derived from n=593 tissue samples. As indicated by their respective names, the rat pan-tissue clock can be applied to DNA methylation profiles from all rat tissues, while the rat brain-, liver-, and blood clocks apply to the corresponding tissue types. We also developed two epigenetic clocks that apply to both human and rat tissues by adding n=850 human tissue samples to the training data. We employed these six clocks to investigate the rejuvenation effects of a plasma fraction treatment in different rat tissues. The treatment more than halved the epigenetic ages of blood, heart, and liver tissue. A less pronounced, but statistically significant, rejuvenation effect could be observed in the hypothalamus. — The treatment was accompanied by progressive improvement in the function of these organs as ascertained through numerous biochemical/physiological biomarkers and behavioral responses to assess cognitive functions. Cellular senescence, which is not associated with epigenetic aging, was also considerably reduced in vital organs. Overall, this study demonstrates that a plasma-derived treatment markedly reverses aging according to epigenetic clocks and benchmark biomarkers of aging.”
Because of the high relevancy of this publication and Dr. Katcher’s earlier work to the life-extending Mission of this blog, Steve Bus and I have arranged to do a series of video interviews with Dr. Katcher on his thinking and this and his other publications The videos of the first two of these interviews are being edited now. We expect that these interviews will be announced in this blog and be made available to the public very shortly.
The results described in this publication are clearly the results of a YOUNGING program. We think that what is involved is the YOUNGING1.0 program as described here. We cannot know that for sure, however, until we know what the ELIXER activator is and how it works – matters about which Dr. Katcher has been silent to protect his proprietary interest in ELIXER.
2. YOUNGING is real and has been demonstrated in rats, and proceeds as a slow programmed process.
In particular, the paper includes a number of graphical displays and tables showing the results of what I believe to be a YOUNGING1.0 initiated by IV administration of the ELIXER substance.
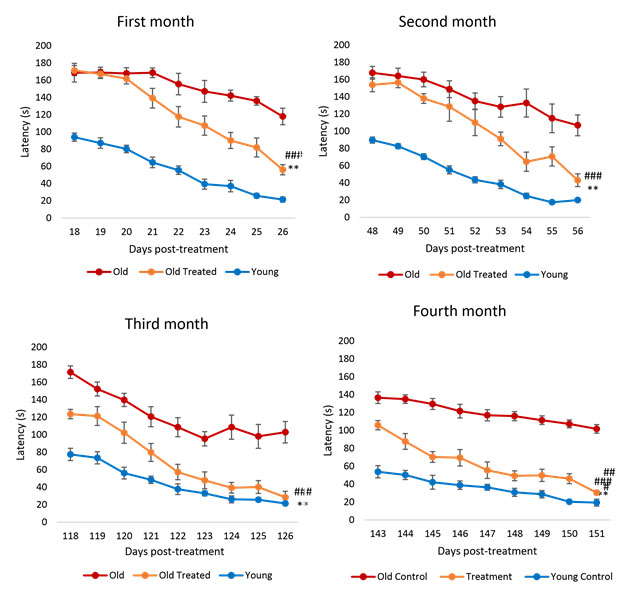
The above chart shows performance of rats in maze running.in terms of latency (time taken) over a 4-month time frame. Since each rat month is roughly equivalent to 2.5 human years, the time frame of 4 months of these charts is equivalent to 10 human years
Focusing on the blue lines for young untreated rats, you see that these rats always go through the maze the quickest, and that for each period there is a downward slope in the line representing rats learning about the maze. The red lines are for untreated older control-group rats. These curves also show a downward slope representing learning but they are far outperformed by the younger rats. The orange curves are for older rats treated with ELIXER. Starting on about day 20 their performance starts to beat that of the control group aged rats. These rats perform relatively better and better over the four month period, ending up being close to as good as the younger rats. These charts illustrate that the YOUNGING1.0 is real and that the YOUNGING1.0 does not happen all at once but proceeds slowly as a program.
There are several other charts of interest in this publication. For example, these charts relate to oxidative stress:
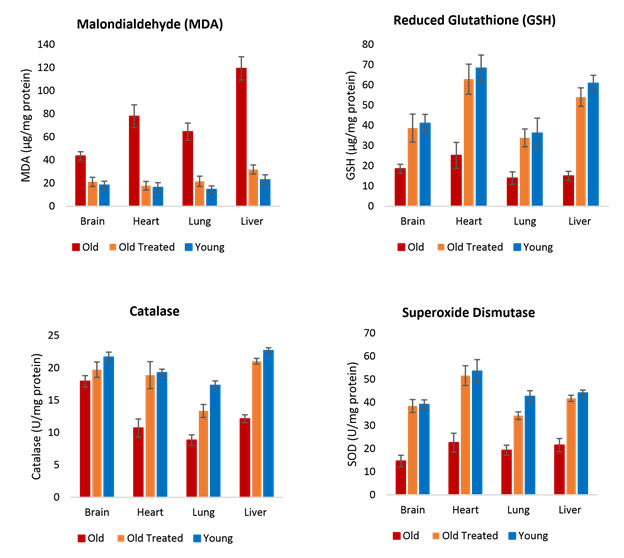
In each case the condition of the treated old rats approaches that of the younger rats, in many but not all cases ending up almost as good. The charts also illustrate that the impacts of the YOUNGING treatment vary by organ and measurement.
There are many other charts in the publication illustrating these and other points I make here.
3. Studies related to hetrochronic parabiosis
There is a rich history of studies demonstrating aspects of YOUNGING via hetrochronic parabiosis. Those studies involved surgically linking the bloodstreams of an older and a blood-type compatible younger mouse, and measuring a number of the resulting “objective” indictors of age in both mice. In short, the result of the connection appeared to be that the older mouse gets biologically younger and the younger mouse gets biologically older. For the older mouse, an astounding demonstration of age reversal. See also these publications.
A recent May 30, 2020 paper by Irina and Michael Conboy with 10 other authors is Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. A principal conclusion of this study paper, appearing also in other Conboy publications is that there are pro-aging factors in the bloodstream plasma of older animals, and reducing the concentration of these may be a more impactful YOUNGING intervention than transferring in plasma with young blood factors from young animals. They report that simply replacing half the plasma in an older mouse with a solution containing albumins is enough to kick off a powerful YOUNGING process in these mice.
“Heterochronic blood sharing rejuvenates old tissues, and most of the studies on how this works focus on young plasma, its fractions, and a few youthful systemic candidates. However, it was not formally established that young blood is necessary for this multi-tissue rejuvenation. Here, using our recently developed small animal blood exchange process, we replaced half of the plasma in mice with saline containing 5% albumin (terming it a “neutral” age blood exchange, NBE) thus diluting the plasma factors and replenishing the albumin that would be diminished if only saline was used. Our data demonstrate that a single NBE suffices to meet or exceed the rejuvenative effects of enhancing muscle repair, reducing liver adiposity and fibrosis, and increasing hippocampal neurogenesis in old mice, all the key outcomes seen after blood heterochronicity. Comparative proteomic analysis on serum from NBE, and from a similar human clinical procedure of therapeutic plasma exchange (TPE), revealed a molecular re-setting of the systemic signaling milieu, interestingly, elevating the levels of some proteins, which broadly coordinate tissue maintenance and repair and promote immune responses. Moreover, a single TPE yielded functional blood rejuvenation, abrogating the typical old serum inhibition of progenitor cell proliferation. Ectopically added albumin does not seem to be the sole determinant of such rejuvenation, and levels of albumin do not decrease with age nor are increased by NBE/TPE. A model of action (supported by a large body of published data) is that significant dilution of autoregulatory proteins that crosstalk to multiple signaling pathways (with their own feedback loops) would, through changes in gene expression, have long-lasting molecular and functional effects that are consistent with our observations. This work improves our understanding of the systemic paradigms of multi-tissue rejuvenation and suggest a novel and immediate use of the FDA approved TPE for improving the health and resilience of older people.”
4. H3K27me2/3 and JMJD3
First of all, don’t be confused because JMJD3 is half the time in the literature referred to by its other name KDM6B. “The KDM6 family includes KDM6A, KDM6B, and UTY. KDM6A (also referred to as UTX) and KDM6B (also referred to as JMJD3)(ref)”
As stated above, H3K27me2 refers to histone bi-methylation and tri-methylation specifically at lysine position 27 on histone 3. And JMJD3 is a highly specific natural demethylator of H3K27me2.
These topics and their relationships have been researched extensively and there is an extensive body of research literature related to them going back more tthan 20 years. I lump these together here because most of the articles dealing with one of them at least touches on the other as well. Here is a short list of key citations. A general observation is that there is widespread acknowledgment of the importance of these subjects in aging and aging processes and diseases, but relatively few publications are focused on aging or general epigenetic state reversal.
That is likely because most of this research has been in the context of cancers and disease processes. For example, the publication Roles of H3K27me3 Demethylase JMJD3 in Inflammation and Cancers: “Histone demethylation is an important part of epigenetic modifications, involving in multiple physiological and pathophysiological processes such as proliferation, differentiation, senescence, apoptosis, reprogramming and so on. JmjC domain-containing protein D3 (JMJD3, also called KDM6B) specifically demethylates lysine 27 on histone H3 (H3K27me3), a repressive epigenetic mark, therefore modulating the expression of target genes. JMJD3 can be strongly and quickly induced by various inflammatory stimuli and cellular stresses, and can enhance pro-inflammatory reactions as well as anti-inflammatory reactions by targeting diverse transcription factors in gene promoters and bodies. Additionally, JMJD3 has a dual effect on many types of cancers through binding to promoters of oncogenes or suppressor genes. As is known to us all, in the occurrence and development of various diseases including inflammation and cancer, JMJD3 plays a crucial role, which has triggered a research boom among numerous scholars over the years. In this review, we primarily focused on the roles of JMJD3 in inflammation and cancers, and briefly discussed its application prospect, laying a theoretical foundation for further research and providing a train of thought for the prevention and treatment of related diseases.”
These factors are involved in multiple growth-and development pathways, and expression of key genes related to cell differentiation, cell senescence and cancer promotion. An example is the 2019 publication Jumonji C Demethylases in Cellular Senescence (JDJM3 is a Jumoni-domain demethylase, probably the one most studied). “Senescence is a stable cell cycle arrest that is either tumor suppressive or tumor promoting depending on context. Epigenetic changes such as histone methylation are known to affect both the induction and suppression of senescence by altering expression of genes that regulate the cell cycle and the senescence-associated secretory phenotype. A conserved group of proteins containing a Jumonji C (JmjC) domain alter chromatin state, and therefore gene expression, by demethylating histones. Here, we will discuss what is currently known about JmjC demethylases in the induction of senescence, and how these enzymes suppress senescence to contribute to tumorigenesis.”
The publication Critical role of histone demethylase Jmjd3 in in the regulation of CD4+ T cell differentiation is important for understanding the operation of the adaptive immune system. “Epigenetic factors have been implicated in the regulation of CD4+ T cell differentiation. Jmjd3 plays a role in many biological processes, but its in vivo function in T cell differentiation remains unknown. Here, we report that Jmjd3 ablation promotes CD4+ T cell differentiation into Th2 and Th17 cells in the small intestine and colon, and inhibits T cell differentiation into Th1 cells under different cytokine-polarizing conditions and in a Th1-dependent colitis model. Jmjd3 deficiency also restrains the plasticity of the conversion of Th2, Th17 or Treg cells to Th1 cells. The skewing of T cell differentiation is concomitant with changes in the expression of key transcription factors and cytokines. H3K27me3 and H3K4me3 levels in Jmjd3-deficient cells are correlated with altered gene expression through interactions with specific transcription factors. Our results identify Jmjd3 as an epigenetic factor in T cell differentiation via changes in histone methylation and target gene expression.”
5. Inflammation and many stresses activate JMJD3 naturally so as to suppress H3K27me2/3.
The publication Roles of H3K27me3 Demethylase JMJD3 in Inflammation and Cancers points this out. “Histone demethylation is an important part of epigenetic modifications, involving in multiple physiological and pathophysiological processes such as proliferation, differentiation, senescence, apoptosis, reprogramming and so on. JmjC domain-containing protein D3 (JMJD3, also called KDM6B) specifically demethylates lysine 27 on histone H3 (H3K27me3), a repressive epigenetic mark, therefore modulating the expression of target genes. JMJD3 can be strongly and quickly induced by various inflammatory stimuli and cellular stresses, and can enhance pro-inflammatory reactions as well as anti-inflammatory reactions by targeting diverse transcription factors in gene promoters and bodies. Additionally, JMJD3 has a dual effect on many types of cancers through binding to promoters of oncogenes or suppressor genes. As is known to us all, in the occurrence and development of various diseases including inflammation and cancer, JMJD3 plays a crucial role, which has triggered a research boom among numerous scholars over the years. In this review, we primarily focused on the roles of JMJD3 in inflammation and cancers, and briefly discussed its application prospect, laying a theoretical foundation for further research and providing a train of thought for the prevention and treatment of related diseases. — Histone modifications can alter structures and functions of genome, but the exact mechanisms remain enigmatic1. Histone methylation is dynamically regulated via methyltransferases and demethylases2. JmjC domain-containing protein D3 (JMJD3, also called KDM6B) is a member of the histone demethylase family, whose C-terminus with JmjC domain catalyzes demethylation, while the N-terminus with basic amino-acids clusters is responsible for nuclear placement3. JMJD3 and the ubiquitously transcribed X-chromosome tetratricopeptide repeat protein (UTX, also called KDM6A) have been identified to specifically demethylate H3K27me2/3, playing key roles in the epigenetic regulation of gene expression2. Seemingly, compared with UTX, JMJD3 is highly regulated at the transcriptional level and more susceptible to various stimuli like differentiation inducers and stress signals2. — The pivotal roles of JMJD3 and relevant mechanisms have been extensively studied for their involvement in cellular proliferation, differentiation, senescence and apoptosis4. From the perspective of tissue responses, JMJD3 mainly embodies in embryonic development, immune system, inflammation, neurodegenerative diseases and tumorigenesis5, 6. Herein, we primarily concentrate on the roles of JMJD3 in inflammation and cancer, aiming at laying a theoretical foundation for further research and providing a train of thought for the prevention and treatment of related diseases.”
6. JMJD3 plays a significant role in regulating CD4+7 CELLS, a matter relevant to development of treatments for cancers, infectious and autoimmune diseases
This is laid out in the 2014 publication Critical role of histone demethylase Jmjd3 in the regulation of CD4+T-cell differentiation. “Epigenetic factors have been implicated in the regulation of CD4+T-cell differentiation. Jmjd3 plays a role in many biological processes, but its in vivo function in T-cell differentiation remains unknown. Here we report that Jmjd3 ablation promotes CD4+T-cell differentiation into Th2 and Th17 cells in the small intestine and colon, and inhibits T-cell differentiation into Th1 cells under different cytokine-polarizing conditions and in a Th1-dependent colitis model. Jmjd3 deficiency also restrains the plasticity of the conversion of Th2, Th17 or Treg cells to Th1 cells. The skewing of T-cell differentiation is concomitant with changes in the expression of key transcription factors and cytokines. H3K27me3 and H3K4me3 levels in Jmjd3-deficient cells are correlated with altered gene expression through interactions with specific transcription factors. Our results identify Jmjd3 as an epigenetic factor in T-cell differentiation via changes in histone methylation and target gene expression. — A growing body of evidence suggests that CD4+Th cells play a central role in initiating and maintaining immune responses against cancer and infectious and autoimmune diseases8,9,10,11. Dysregulation of CD4+ T-cell differentiation is associated with various autoimmune and inflammatory diseases, including myelodysplastic syndromes and systemic lupus erythematosus12,13,14. Regulation of CD4+T-cell differentiation is essential to maintain the appropriate balance among CD4+T-cell subsets to support immune homeostasis and prevent autoimmunity. Therefore, understanding the mechanisms regulating and controlling CD4+T-cell differentiation into various subsets is of critical importance to develop innovative treatments. CD4+T-subset differentiation is tightly regulated by many signalling molecules, including signal transducer and activator of transcription (STAT) proteins, IFN regulatory factor 4 (IRF4) and runt-related transcription factor 1 (refs 2, 15). In addition to transcription factors, recent studies have shown that epigenetic factors control the specificity and plasticity of T-cell subsets16,17,18,19. These transcriptional factors and epigenetic regulators bind to both shared and cell-specific regulatory regions (promoters and enhancers) among various T-cell lineages and thereby, positively or negatively regulate gene expression depending upon the cofactors present20. — Jmjd3 (also known as KDM6B) was found to catalyse the demethylation of H3K27me2/3 in vitro27,28,29,30,31,32. Jmjd3 is induced by vitamin D and proinflammatory stimuli in macrophages and is required for Ink4a-Arf, Nodal and Irf4 expression in fibroblasts, mouse embryonic stem cells and macrophages, respectively33,34,35,36,37.
7. A thorough understanding of JMJD3 and H3Kme2/3 requires grappling with many complex factors
Underneath the simple facts that JMJD3 demethylates H3K27me2/3 producing an amazing number of biological impacts, like everywhere else in biology there is a crazy-making number of details to sort through. For example, there are several other histone demethylases that demethylate H3K27me2/3 . These points are illustrated in the 2020 document Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism. “Significance T cells control many immune functions, with Th17 cells critical in regulating inflammation. Following activation, T cells undergo metabolic reprogramming and utilize glycolysis to increase the ATP availability. Epigenetic mechanisms controlling metabolic functions in T cells are currently not well-defined. Here, we establish an epigenetic link between the histone H3K27me3 demethylases KDM6A/B and the coordination of a metabolic response. Inhibition of KDM6A/B leads to global increases in the repressive H3K27me3 histone mark, resulting in down-regulation of key transcription factors, followed by metabolic reprogramming and anergy. This work suggests a critical role of H3K27 demethylase enzymes in maintaining Th17 functions by controlling metabolic switches. Short-term treatment with KDM6 enzyme inhibitors may be useful in the therapy of chronic inflammatory diseases. T helper (Th) cells are CD4+ effector T cells that play a critical role in immunity by shaping the inflammatory cytokine environment in a variety of physiological and pathological situations. Using a combined chemico-genetic approach, we identify histone H3K27 demethylases KDM6A and KDM6B as central regulators of human Th subsets. The prototypic KDM6 inhibitor GSK-J4 increases genome-wide levels of the repressive H3K27me3 chromatin mark and leads to suppression of the key transcription factor RORγt during Th17 differentiation. In mature Th17 cells, GSK-J4 induces an altered transcriptional program with a profound metabolic reprogramming and concomitant suppression of IL-17 cytokine levels and reduced proliferation. Single-cell analysis reveals a specific shift from highly inflammatory cell subsets toward a resting state upon demethylase inhibition. The root cause of the observed antiinflammatory phenotype in stimulated Th17 cells is reduced expression of key metabolic transcription factors, such as PPRC1. Overall, this leads to reduced mitochondrial biogenesis, resulting in a metabolic switch with concomitant antiinflammatory effects. These data are consistent with an effect of GSK-J4 on Th17 T cell differentiation pathways directly related to proliferation and include regulation of effector cytokine profiles. This suggests that inhibiting KDM6 demethylases may be an effective, even in the short term, therapeutic target for autoimmune diseases, including ankylosing spondylitis.” So here we have the suggestion that inhibiting JMJD3 rather than promoting it may be appropriate for some with autoimmune conditions. The drug GSK-J4 does this.
8. JMJD3 inhibits natural reprogramming of normal somatic cells into stem cells via the Yamanaka OSKM FACTORS.
I (Vince) have thought that an important aspect of YOUNGING was a natural body process where senescence cell IL-6 cytokine signaling would cause upgrading of the Yamanaka factors in some proximate cells causing them to regress epigenetically by de-differentiation (that is YOUNGER), but only part of the way so they would retain memory of their cell lineage. There they would become progenitor cells capable of differentiation into new cells to replace the senescent ones. I described that process in detail in my 2018 blog entry AGING, CELL AND TISSUE REPAIR, RENEWAL AND REGENERATION, INFLAMMATION AND THE SASP. However JDJM3 promotes differentiation and inhibits de-differentiation so it inhibits that process. That is pointed out, for example in the 2016 publication The localization of histone H3K27me3 demethylase Jmjd3 is dynamically regulated. “Jmjd3 is required for cellular differentiation and senescence, and inhibits the induction of pluripotent stem cells by demethylating histone 3 lysine 27 trimethylation (H3K27me3).” Further, this article points out the nuclear translocation is a prerequisite for JMJD3 to exercise its histone demethylation capabilities.
- The 2015 article JMJD3 as an epigenetic regulator in development and disease offers a diagram illustrates the point.
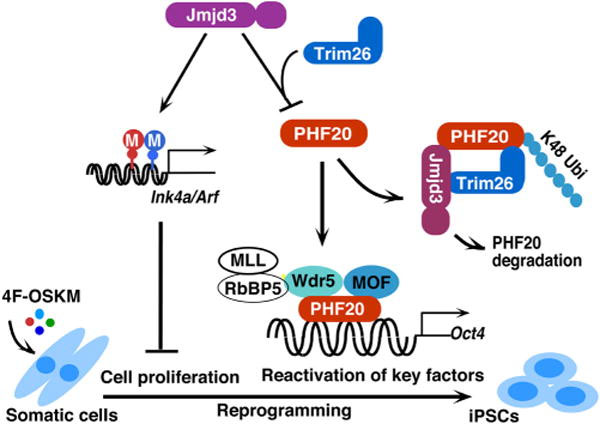
“A working model to illustrate how JMJD3 negatively regulates cellular reprogramming through demethylase-dependent and independent pathways. JMJD3 upregulates Ink4a/Arf and p21 by removal of H3K27 methylation through its H3K27me2/3 demethylase activity. Increased amounts of Ink4a and Arf induce cell senescence or apoptosis and reduce cell proliferation, which leads to decrease in efficiency and kinetics of reprogramming. Importantly, JMJD3 protein also targets PHF20 for ubiquitination and degradation by recruiting an E3 ligase Trim26 in an H3K27 demethylase activity-independent manner. PHF20 is required for the reactivation of key core reprogramming factors such as Oct4 through interaction with WDR5. Thus, downregulation of PHF20 protein by JMJD3 leads to the inhibition of reprogramming efficiency”
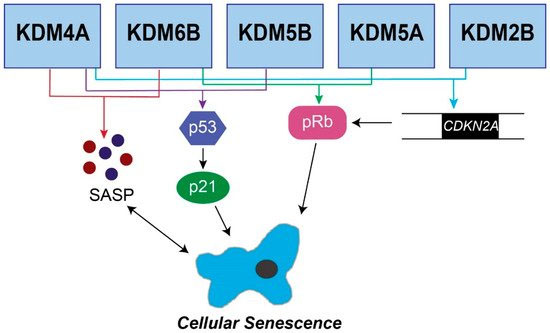
9. JMJD3 is an important part of the cell senescence pathway
The 2019 article Jumonji C Demethylases in Cellular Senescence reports on this. “Senescence is a stable cell cycle arrest that is either tumor suppressive or tumor promoting depending on context. Epigenetic changes such as histone methylation are known to affect both the induction and suppression of senescence by altering expression of genes that regulate the cell cycle and the senescence-associated secretory phenotype. A conserved group of proteins containing a Jumonji C (JmjC) domain alter chromatin state, and therefore gene expression, by demethylating histones. Here, we will discuss what is currently known about JmjC demethylases in the induction of senescence, and how these enzymes suppress senescence to contribute to tumorigenesis.” This diagram from the publication illustrates the mechanism. Recall that KDM6B is an alias for JMJD3.
10. Gene regulation shift around age 24: time of “loss of youth”
In SECTION 1 we asserted that “there is a major shift in epigenetic gene regulation in humans typically centered around age 24, where numerous growth and protective genes are down-regulated and inflammatory and pro-aging genes are upregulated.” The process is highly evolutionarily conserved. How it works in nematodes was laid out in a 2015 paper by Morimoto and his colleague. Repression of the Heat Shock Response Is a Programmed Event at the Onset of Reproduction “The heat shock response (HSR) is essential for proteostasis and cellular health. In metazoans, aging is associated with a decline in quality control, thus increasing the risk for protein conformational disease. Here, we show that in C. elegans, the HSR declines precipitously over a 4 hr period in early adulthood coincident with the onset of reproductive maturity. Repression of the HSR occurs due to an increase in H3K27me3 marks at stress gene loci, the timing of which is determined by reduced expression of the H3K27 demethylase jmjd-3.1. This results in a repressed chromatin state that interferes with HSF-1 binding and suppresses transcription initiation in response to stress. The removal of germline stem cells preserves jmjd-3.1 expression, suppresses the accumulation of H3K27me3 at stress gene loci, and maintains the HSR. These findings suggest that competing requirements of the germline and soma dictate organismal stress resistance as animals begin reproduction.” It is fascinating that the methylation and loss of gene regulation happens so quickly once adulthood arrives– in 4 hours for nematodes, around 25 years of age for humans.
Highlights
- Stress responses are rapidly repressed at the onset of egg laying in C. elegans
- Transcriptional repression at stress response genes is due to increased H3K27me3
- Reduced jmjd-3.1 expression underlies increased H3K27me3 at stress response genes
- Repression of stress responses is regulated by germline stem cells”
The graphical abstract applies tells the story clearly
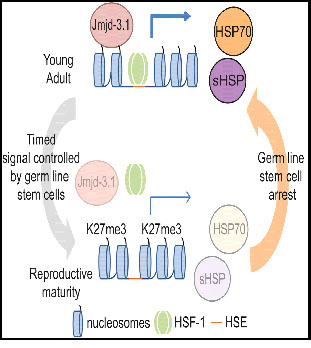
”It is thought that the progressive dysregulation of stress response pathways contributes to aging in metazoans. Here, Labbadia and Morimoto demonstrate that stress responses are rapidly repressed early in C. elegans adulthood as part of a genetically programmed event controlled by germ line stem cells through alterations in chromatin accessibility.”
Recall that a primary function of the heat shock response and upgrading of heat shock proteins like HSP70 is keeping proteins folded correctly. However, the heat shock HSF-1 response plays a role in the activation of many cancers, so downgrading this in early adulthood could be an anti-cancer mechanism conserved by evolution.
Morimoto and his team subsequently published a 2017 paper Mitochondrial stress restores the heat shock response and prevents proteostasis collapse during aging. There, they found that “mitochondrial perturbation”, a specific kind of mitochondrial electron transport chain manipulation, restored more youthful JMJD3 and H3K27me3 expressions. “In Caenorhabditis elegans, the programmed repression of the heat shock response (HSR) accompanies the transition to reproductive maturity, leaving cells vulnerable to environmental stress and protein aggregation with age. To identify the factors driving this event, we performed an unbiased genetic screen for suppressors of stress resistance and identified the mitochondrial electron transport chain (ETC) as a central regulator of the age-related decline of the HSR and cytosolic proteostasis. Mild downregulation of ETC activity, either by genetic modulation or exposure to mitochondria-targeted xenobiotics, maintained the HSR in adulthood by increasing HSF-1 binding and RNA polymerase II recruitment at HSF-1 target genes. T his resulted in a robust restoration of cytoplasmic proteostasis and increased vitality later in life, without detrimental effects on fecundity. We propose that low levels of mitochondrial stress regulate cytoplasmic proteostasis and healthspan during aging by coordinating the long-term activity of HSF-1 with conditions preclusive to optimal fitness.”
A 2016 study by a different team,Two Conserved Histone Demethylases Regulate Mitochondrial Stress-Induced Longevity, addresses the question of whether the “mitochondrial perturbation” of the electron transfer chain just mentioned is an alternative path to greater youthfulness that doesn’t include or impact the JMJD3/H3K27me3 combination? The bullet points below are the “Results” section subtitles of the study.
- “Mitochondrial ETC-mediated longevity requires the histone lysine demethylases jmjd-1.2 and jmjd-3.1
- There are overlapping temporal requirements of JmjC demethylase activity and ETC-mediated longevity
- JMJD-1.2 and JMJD-3.1 regulate the mitochondrial Unfolded Protein Response (UPRmt)
- Overexpression of jmjd-1.2 or jmjd-3.1 is sufficient for lifespan extension and UPRmt induction
- The UPRmt is a genetic requirement for JmjC demethylase-mediated longevity
- JMJD-1.2 and JMJD-3.1 Overexpression Recapitulates the Transcriptional Response to Mitochondrial Stress
- Mammalian PHF8 and JMJD3 Correlate with Lifespan and UPRmt Activation”
11. Nicotinamide riboside, the mitochondrial UPR response and extending mammalian longevity
The 2016 publication NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice is among those reporting on this issue. “Adult stem cells (SCs) are essential for tissue maintenance and regeneration yet are susceptible to senescence during aging. We demonstrate the importance of the amount of the oxidized form of cellular nicotinamide adenine dinucleotide (NAD(+)) and its effect on mitochondrial activity as a pivotal switch to modulate muscle SC (MuSC) senescence. Treatment with the NAD(+) precursor nicotinamide riboside (NR) induced the mitochondrial unfolded protein response and synthesis of prohibitin proteins, and this rejuvenated MuSCs in aged mice. NR also prevented MuSC senescence in the mdx (C57BL/10ScSn-Dmd(mdx)/J) mouse model of muscular dystrophy. We furthermore demonstrate that NR delays senescence of neural SCs and melanocyte SCs and increases mouse life span. Strategies that conserve cellular NAD(+) may reprogram dysfunctional SCs and improve life span in mammals.”
12. H3K27me3 and its methylation/demethylation status plays a similar role in the transition from youth to adulthood in plants as in animals
There are many studies about this. A fascinating collection of diagrams showing numerous roles of H3K27me3 in plants can be found here. For example this diagram shows loss of H27me markers in the transition from plant juvenile phase to adult phase, essentially the same thing we described here as happening in nematodes and humans. The implication is that this is a very basic transition applicable to the life cycles and aging of most if not all living things. And undoing it may well be the key to YOUNGING.
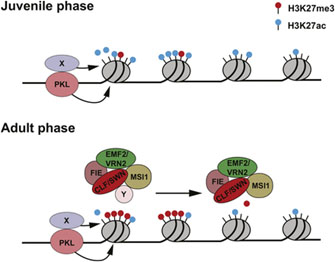
There will likely be much much more to write about all of this in following blogs in this YOUNGING series.


Very encouraging. However, when it comes to many of the aspects out of the cells control, like stiffening of the ECM due to protein cross-linking, I am somewhat more skeptical. Your cells may act like a 20 y.o, (methylation wise) but if all of your connective tissue like skin, tendons, ligaments and joints feel like a 90 year old, whats the point? You may be biologically alive, but if you can no longer enjoy a sprint or go hiking I’d rather just pass out at the age of 90.
Pingback: Introduction to the YOUNGING Series – Emerging Aging Reversal Strategies and Treatments - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
I received this comment from AKSHAY SANGHVI, who gave me permission to publish it as a comment to this blog entry from him.
I have always admired your analytics and this one too is interesting. I agree with you on how important is Morimoto’s discovery – I too covered it in my blog couple of years ago. It shows that aging is programmed and begins its cascade just after puberty. You have deduced the importance of jmjd3 and its demthylayion of H3K27me2.
My thoughts: ENCODE data shows that 75% of our genome is transcribed but only 3% of that is translated into proteins. We are still in the dark of the roles and actions of the balance 72%. I suspect majority of them would be regulatory and that this transcriptome changes over our life cycle determining major changes to our cellular proteome and secretome. There is tremendous amount of cross talk between plasma factors, organ specific secretome and cellular proteome and other transcription factors. Our ECM our bones our hypothalamus every tissue and organ seems to play its instrument in this orchestra which manages and diffuses the life cycle changes including those related to aging. Plasma plays an important role in ensuring that these changes are more or less evenly distributed. Most of our scientific research has focused on cells as it houses the DNA but as Harold and another scientist I admire a lot: Mina Bissell both have shown that the environment too governs the cell. She showed that a cancerous cell reversed to a normal cell when housed in a young ECM. This is a very important discovery embellishing our approach. We have hacked one section of this orchestra with young factors. Our advantage is that it is the medium that diffuses the changes across other organs and tissues and their cells. Which is why we have seen such a systemic response versus interventions on individual genes or certain transcription activators.
We are using Nature’s own tools for younging. This approach should not cause cancer unlike interventions like OSKM reprogramming for example. In fact as Dr. Bissell has shown our intervention could reverse some age onset cancers.
Thanks for contacting Akshay on this topic and for posting his reply on this blog.
I agree on many of his viewpoints and arguments, however we know that stiffening of the ECM is one of the major symptoms of aging. Protein cross-linking is an ongoing process and inevitable, unfortunately.
Currently, I am not aware of any botanical extract or drug, which can safely reach all tissues across the human body and break up cross linked proteins. Also, I haven’t seen any scientific evidence that a rejuvenated cell will be able to affect already crosslinked proteins.
I sincerely hope that Harold’s and Akshay’s Elixir will deliver on its promise. If so, it will be a gigantic step for human anti-aging interventions.
I’m just a layman here, but I think people get hung up in the wrong question sometimes. You have to go beach to root cause analysis and keep adding why? In this case does the body have the ability to repair cross-linked proteins. My shirt look says yes. Is that process robust in 20 year olds? If it so then the question is: why doesn’t it Repair the damage as well as you get older.
Pingback: MORE ON YOUNGING1.0 – THE EMERGING AGING REVERSAL STRATEGY - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: YGD YOUNGING1.0 PART 4: UNDERLYING MECHANISMS OF YOUNGING 1.0. HSC STEM CELL DIFFERENTIATION - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: YGE YOUNGING 1.0 PART 5: PRACTICAL INITIATION OF YOUNGING 1.0 VIA HYPOXIC INTERVENTIONS - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: Two faces of Life Extension - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Vince
I loved the article and your dedication. I am 71 and in good health and am constantly on the prowl for strategies to stay that way. Here are a few random thoughts based on reading I have done.
The comment about replacing half the blood with plasma intrigued me. Doing so would cut the free iron content of the blood by 50%. Blood iron is usually measure by the bound form but is a surrogate measure for free iron. Blood donors are often noted to be generally healthier and women’s rates of heart disease go up in menopause. Elevated ferritin levels are correlated with a lot of aging diseases. The book “Dumping Iron” by P.D. Mangan goes into this in some detail. Another thought is levels of boron. According to Dr. Walter Last, Boron deficiency is linked to arthritic conditions and cancer, among other things.
https://ia804507.us.archive.org/33/items/borax-conspiracy/Borax%20Conspiracy.pdf
I personally have put people on boron supplements and seen improvements in inflammation. Finally, although infusions of younger blood may indeed ward off aging, I would caution that such practices cannot be ethically recommended as it is not practical on any large scale, and the temptation for abuse by unscrupulous is real.
Mike