The intent of this blog entry is to present a sampler of recent research relating to the mTOR pathway and the effects of rapamycin, focusing on recent and longevity-related results not covered in previous blog entries.
Background
Of course mTOR stands for mammalian target of rapamycin and the drug rapamycin inhibits the mTOR pathway. I have already written several blog entries related to the mTOR pathways. For a general introduction I suggest readers review Longevity genes, mTOR and lifespan. Other relevant past blog entries include Viva mTOR! Caveat mTOR! and More mTOR links to aging theories .
In my treatise one of the advanced “candidate” aging theories is Increasing mTOR signaling which happens with aging.” There, I described how the mTOR pathways appears to be highly conserved across species and how in primitive species as well as mice, inhibition of mTOR signaling is an effective strategy for extending longevity as well as addressing many disease processes.
The blog entry AMPK and longevity discusses the intimate relationship between the AMPK and mTOR pathways, and how activation of the former results in inhibition of the latter. The second part of the blog entry Curcumin, cancer and longevity contains a discussion of how curcumin inhibits mTOR expression and how feeding of rapamycin to mice is life-extending in mice. Other than for the material in this background section, my intent in this blog entry is to cover material not already covered.
I have previously written “Mammalian target of rapamycin (mTOR) is a protein encoded in humans by the FRAP1 gene. As the name suggests, mTOR is targeted by the immunosuppressive drug rapamycin, a drug used clinically to treat graft rejection and restenosis and being tested as a treatment for autoimmune diseases. “The mTOR pathway integrates signals from nutrients, energy status and growth factors to regulate many processes, including autophagy, ribosome biogenesis and metabolism(ref, ref).” The mTOR pathway is “a central controller of cellular and organism growth that integrates nutrient and hormonal signals, and regulates diverse cellular processes(ref).”
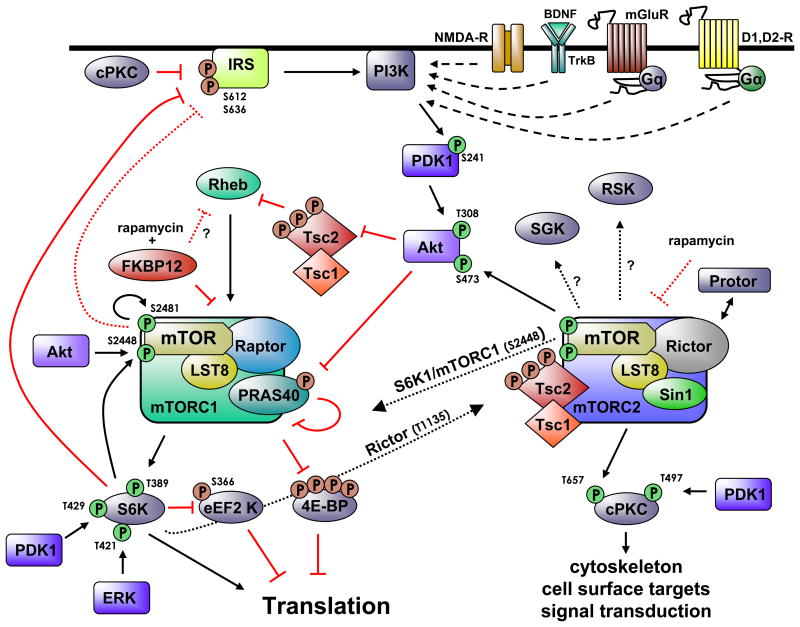
This diagram showing some of the key pathways related to mTOR appeared in the publication mTOR signaling: at the crossroads of plasticity, memory and disease:
As you may note, there are two related pathways involved commonly referred to as mTORC1 and mTORC2. mTORC1 is associated with Raptor. See The Mammalian Target of Rapamycin (mTOR) Partner, Raptor, Binds the mTOR Substrates p70 S6 Kinase and 4E-BP1 through Their TOR Signaling (TOS) Motif . “Raptor (regulatory associated protein of mTOR) is a recently identified mTOR binding partner that also binds p70S6k and 4E-BP1 and is essential for TOR signaling in vivo” mTORC2 is in turn associated with Rictor, a different adaptor protein. Some inhibitors of mTOR affect only one of these pathways; others affect both. Likewise, disease processes may depend on interactions among the two pathways. For example, consider what is reported in the December 2010 publication Dual Inhibition of mTORC1/mTORC2 Induces Apoptosis of Mantle Cell Lymphoma by Preventing Rictor Mediated AKTS473 Phosphorylation by Potentiating AKT2-PHLPP1 Association. mTORC2 is largely resistant to rapamycin(ref).
Clearly, the mTOR-related pathways are very complex and relate centrally to many critical biological functions.
mTOR and skeletal muscle
There is a body of literature going back some time concerned with the role of mTOR and other signaling pathways in the health and aging of skeletal muscle. See, for example, Skeletal muscle hypertrophy is regulated via AKT/mTOR pathway. “Skeletal muscle atrophies with disuse while with increased use and increased load skeletal muscle exhibits hypertrophy, with an increase in the size of existing muscle fibers. One signaling pathway involved in regulating skeletal muscle atrophy and hypertrophy is the AKT/mTOR pathway (see mTOR pathway). The mTOR pathway activity increases in response to muscle activity during hypertrophy and decreases in activity during atrophy. Blocking this pathway genetically or with the mTOR inhibitor rapamycin blocks hypertrophy and genetic activation of the pathway induces hypertrophy.”
mTOR and exercise
Consistent with the above, it appears that physical activity downregulates mTOR/S6K1 signaling and downregulates IRS-1 serine phosphorylation, at least in rat skeletal muscle. See the 2010 publication Effects of Physical Actyivity and Nutritional Intake on Skeletal Muscle Protein Turnover and Cellular Signaling. So, could downregulation of mTOR be a pathway through which exercise increases longevity? It is an interesting conjecture.
Potential medical uses of rapamycin
Rapamycin is also known as sirolimus. “It is marketed under the trade name Rapamune by Wyeth.” Rapamycin (sirolimus) is already in use as an immunosuppressant to prevent rejection of kidney transplants and as a treatment for psoriasis(ref).”
Possible new medical applications related to modulating the mTOR pathway
For several disease processes including sarcopenia, spinal cord injuries and epilepsy, researchers have been expressing hope that effective therapeutic interventions might be based on modulation of mTOR signaling.
mTOR and sarcopenia
“Sarcopenia (from the Greek meaning “poverty of flesh”) is the degenerative loss of skeletal muscle mass and strength associated with aging (0.5-1% loss per year after the age of 25). Sarcopenia is a component of the frailty syndrome(ref).”
The 2011 publication Aging impairs contraction-induced human skeletal muscle signaling and protein synthesis reports “Sarcopenia, the loss of skeletal muscle mass during aging, increases the risk for falls and dependency. Resistance exercise (RE) training is an effective treatment to improve muscle mass and strength in older adults, but aging is associated with a smaller amount of training-induced hypertrophy. — This may be due in part to an inability to stimulate muscle-protein synthesis (MPS) after an acute bout of RE. We hypothesized that older adults would have impaired mammalian target of rapamycin complex (mTORC)1 signaling and MPS response compared with young adults after acute RE. — Conclusions: We conclude that aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. These age-related differences may contribute to the blunted hypertrophic response seen after resistance-exercise training in older adults, and highlight the mTORC1 pathway as a key therapeutic target to prevent sarcopenia.”
The 2010 publication mTOR signaling as a target of amino acid treatment of the age-related sarcopenia reflects a hope and expectation that interventions in the mTOR pathway might be used to avert sarcopenia in elderly people. “Sarcopenia is an age-related structural and functional impairment of skeletal muscle leading to loss of strength, contractile capacity and endurance. Among factors implicated in sarcopenia, deregulation of muscle protein synthesis (MPS) has frequently been reported. Thus, the attempts aiming at identifying possible countermeasures to sarcopenia require consideration of a complex coordinated interaction of factors contributing to the balance between protein synthesis and breakdown and the identification of several regulators on their function. We will focus here on the signaling pathways controlling protein synthesis in skeletal muscle, specifically on one of the downstream effectors of the kinase Akt/PKB, the mammalian target of rapamycin (mTOR) kinase which is now recognized as a key regulator of cell growth and a pivotal sensor of nutritional status over the lifespan. Dysfunction of mTOR signaling in the elderly and its potential role as a target of amino acids in the treatment of age-related sarcopenia will be discussed.”
mTOR and spinal cord injury
The 2010 publication ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats suggests that interventions in the Akt/mTOR/p70S6K signaling pathway may improve recovery prospects after spinal cord injuries. “The protein kinase B (Akt)/mammalian target of rapamycin (mTOR)/p70 ribosomal S6 protein kinase (p70S6K) signaling pathway, as a central controller of cell growth, proliferation, survival, and differentiation in response to extracellular signals, growth factors, nutrient availability, energy status of the cell, and stress, has recently gained attention in neuroscience. The effects of this signaling pathway on repair of spinal cord injury (SCI), however, have not been well elucidated. ATP is increasingly recognized as an important regulator of signal transduction pathways, and plays important roles in functional recovery after nervous system injury. In the present study, we examined the ATP-induced changes of the Akt/mTOR/p70S6K signaling pathway in injured spinal cord of adult rats and potential therapeutic effects of this pathway on SCI-induced locomotor dysfunction. SCI was produced by extradural weight-drop using modified Allen’s stall with damage energy of 50 g-cm force. The rats were divided into four groups: SCI plus ATP, SCI plus saline, SCI plus ATP and rapamycin, and sham-operated. Using immunostaining studies, Western blot analyses and real-time qualitative RT-PCR analyses, we demonstrated that the Akt/mTOR/p70S6K signaling pathway is present in the injured spinal cord and the expression of its components at the protein and mRNA levels is significantly elevated by exogenous administration of ATP following SCI. We observed the effectiveness of the activated Akt/mTOR/p70S6K signaling pathway in improving locomotor recovery, significantly increasing the expression of nestin, neuronal nuclei (NeuN), neuron specific enolase (NSE), and neurofilament 200 (NF200), and relatively inhibiting excessive reactive astrogliosis after SCI in a rapamycin-sensitive manner. We concluded that ATP injection produced a significant activation of the Akt/mTOR/p70S6K signaling pathway in the injured spinal cord and that enhancement of rapamycin-sensitive signaling produces beneficial effects on SCI-induced motor function defects and repair potential. We suggest that modulation of this protein kinase signaling pathway activity should be considered as a potential therapeutic strategy for SCI.”
mTOR and epilepsy
A number of publications point to inhibition of mTOR signaling as a potential strategy for preventing epileptic seizures. These include the 2008 publications Review Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation and Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex, and the 2009 publication The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy.
The 2009 publication Developing Antiepileptogenic Drugs for Acquired Epilepsy: Targeting the Mammalian Target of Rapamycin (mTOR) Pathway reports “A rational antiepileptogenic strategy is to target primary cell signaling pathways that initially trigger the downstream mechanisms causing epileptogenesis. Recent work implicates the mammalian target of rapamycin (mTOR) pathway as mediating epileptogenesis in a genetic epilepsy, Tuberous Sclerosis Complex (TSC), and suggests that mTOR inhibitors, such as rapamycin, may have antiepileptogenic properties for epilepsy in TSC. As mTOR regulates multiple cellular functions that may contribute to epileptogenesis in general, including ion channel expression, synaptic plasticity, and programmed cell death, mTOR inhibitors might also represent an effective antiepileptogenic therapy for other, more common types of epilepsy, such as acquired epilepsies due to brain injuries.” The 2010 publication Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies relates “The mammalian target of rapamycin (mTOR) pathway represents a logical candidate, because mTOR regulates multiple cellular functions that may contribute to epileptogenesis, including protein synthesis, cell growth and proliferation, and synaptic plasticity. The importance of the mTOR pathway in epileptogenesis is best illustrated by tuberous sclerosis complex (TSC), one of the most common genetic causes of epilepsy. In mouse models of TSC, mTOR inhibitors prevent the development of epilepsy and underlying brain abnormalities associated with epileptogenesis. Accumulating evidence suggests that mTOR also participates in epileptogenesis due to a variety of other causes, including focal cortical dysplasia and acquired brain injuries, such as in animal models following status epilepticus or traumatic brain injury. Therefore, mTOR inhibition may represent a potential antiepileptogenic therapy for diverse types of epilepsy, including both genetic and acquired epilepsies.”
An October 2010 report Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy sounds a largely optimistic note: “Inhibition of mTOR by rapamycin has been shown to suppress seizures in TSC/PTEN genetic models. Rapamycin, when applied immediately before or after a neurological insult, also prevents the development of spontaneous recurrent seizures (epileptogenesis) in an acquired model. In the present study, we examined the mTOR pathway in rats that had already developed chronic spontaneous seizures in a pilocarpine model. We found that mTOR is aberrantly activated in brain tissues from rats with chronic seizures. Furthermore, inhibition of mTOR by rapamycin treatment significantly reduces seizure activity. Finally, mTOR inhibition also significantly suppresses mossy fiber sprouting. Our findings suggest the possibility for a much broader window for intervention for some acquired epilepsies by targeting the mTOR pathway.”
These reports are mostly based on mouse-model experiments involving a genetic TSC-related epilepsy. Another 2010 study report Regulation of cell death and epileptogenesis by the mammalian target of rapamycin (mTOR): A double-edged sword? sounds a note of caution. “Identification of cell signaling mechanisms mediating seizure-related neuronal death and epileptogenesis is important for developing more effective therapies for epilepsy. The mammalian target of rapamycin (mTOR) pathway has recently been implicated in regulating neuronal death and epileptogenesis in rodent models of epilepsy. In particular, kainate-induced status epilepticus causes abnormal activation of the mTOR pathway, and the mTOR inhibitor, rapamycin, can decrease the development of neuronal death and chronic seizures in the kainate model. Here, we discuss the significance of these findings and extend them further by identifying upstream signaling pathways through which kainate status epilepticus activates the mTOR pathway and by demonstrating limited situations where rapamycin may paradoxically increase mTOR activation and worsen neuronal death in the kainate model. Thus, the regulation of seizure-induced neuronal death and epileptogenesis by mTOR is complex and may have dual, opposing effects depending on the physiological and pathological context. Overall, these findings have important implications for designing potential neuroprotective and antiepileptogenic therapies that modulate the mTOR pathway.”
Finally, a 2011 study Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy reports interesting additional insights “Temporal lobe epilepsy is prevalent and can be difficult to treat effectively. Granule cell axon (mossy fiber) sprouting is a common neuropathological finding in patients with mesial temporal lobe epilepsy, but its role in epileptogenesis is unclear and controversial. Focally infused or systemic rapamycin inhibits the mammalian target of rapamycin (mTOR) signaling pathway and suppresses mossy fiber sprouting in rats. We tested whether long-term systemic treatment with rapamycin, beginning 1 d after pilocarpine-induced status epilepticus in mice, would suppress mossy fiber sprouting and affect the development of spontaneous seizures. Mice that had experienced status epilepticus and were treated for 2 months with rapamycin displayed significantly less mossy fiber sprouting (42% of vehicle-treated animals), and the effect was dose dependent. However, behavioral and video/EEG monitoring revealed that rapamycin- and vehicle-treated mice displayed spontaneous seizures at similar frequencies. These findings suggest mossy fiber sprouting is neither pro- nor anti-convulsant; however, there are caveats. Rapamycin treatment also reduced epilepsy-related hypertrophy of the dentate gyrus but did not significantly affect granule cell proliferation, hilar neuron loss, or generation of ectopic granule cells. These findings are consistent with the hypotheses that hilar neuron loss and ectopic granule cells might contribute to temporal lobe epileptogenesis.”
Rapamycin and clinical trials
Although the above applications seem still-removed from clinical practice, a very large number of new specialized uses for rapamycin are in clinical trials. A search of clinicaltrials.gov using the term rapamycin reveals 639 clinical trials in various phases. Many of these relate to the immunosuppressant properties of rapamycin such as for prevention of transplant rejections and graft-host disease. A number relate to use of the substance on stents. Many relate to rapamycin as part of chemotherapy regimens for a large number of cancers. And some are investigating rapamycin for a wide variety of other diseases including Multiple Sclerosis, Polycystic Kidney Diseases, Tuberous Sclerosis; Lymphangioleiomyomatosis, In-Stent Restenosis, Oral Lichen Planus, Systemic Sclerosis, Angiomyolipoma, Diabetic Retinopathy, Aplastic Anemia, Glomerulosclerosis, Autoimmune Active Anterior Uveiti, and Age-Related Macular Degeneration. I conjecture that some or many of these trials will yield positive results and that therefore rapamycin will come into wider clinical use for a number of specialized applications.
Chronic use of rapamycin has not been seriously considered as an anti-aging treatment for humans because of safety concerns. One set of concerns has to do with the drug’s immunosuppressive properties. Another set of concerns has to do with its effects on metabolism.
Chronic rapamycin treatment can result in metabolic derangement
The 2010 publication Chronic Rapamycin Treatment Causes Glucose Intolerance and Hyperlipidemia by Upregulating Hepatic Gluconeogenesis and Impairing Lipid Deposition in Adipose Tissue reports “OBJECTIVE The mammalian target of rapamycin (mTOR)/p70 S6 kinase 1 (S6K1) pathway is a critical signaling component in the development of obesity-linked insulin resistance and operates a nutrient-sensing negative feedback loop toward the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt pathway. Whereas acute treatment of insulin target cells with the mTOR complex 1 (mTORC1) inhibitor rapamycin prevents nutrient-induced insulin resistance, the chronic effect of rapamycin on insulin sensitivity and glucose metabolism in vivo remains elusive. — RESEARCH DESIGN AND METHODS To assess the metabolic effects of chronic inhibition of the mTORC1/S6K1 pathway, rats were treated with rapamycin (2 mg/kg/day) or vehicle for 15 days before metabolic phenotyping. — RESULTS Chronic rapamycin treatment reduced adiposity and fat cell number, which was associated with a coordinated downregulation of genes involved in both lipid uptake and output. Rapamycin treatment also promoted insulin resistance, severe glucose intolerance, and increased gluconeogenesis. The latter was associated with elevated expression of hepatic gluconeogenic master genes, PEPCK and G6Pase, and increased expression of the transcriptional coactivator peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) as well as enhanced nuclear recruitment of FoxO1, CRTC2, and CREB. These changes were observed despite normal activation of the insulin receptor substrate/PI 3-kinase/Akt axis in liver of rapamycin-treated rats, as expected from the blockade of the mTORC1/S6K1 negative feedback loop. — CONCLUSIONS These findings unravel a novel mechanism by which mTORC1/S6K1 controls gluconeogenesis through modulation of several key transcriptional factors. The robust induction of the gluconeogenic program in liver of rapamycin-treated rats underlies the development of severe glucose intolerance even in the face of preserved hepatic insulin signaling to Akt and despite a modest reduction in adiposity.” Alternative inhibitors of rmTOR signaling



lots of those who are looking for an effective anti aging treatment would love this post.
Pingback: New, emerging and potential treatments for cancers: Part 1 – focus on the m/TOR pathway | AGING SCIENCES – Anti-Aging Firewalls