By Vince Giuliano
We are being exposed to a lot more blue and ultraviolet light in recent years, especially at night. This is due to 1. fluorescent bulbs replacing incandescent bulbs in homes and workspaces, especially “daytime” spectrum bulbs, 2. Flat LED TV screens replacing the old big-box tube fluorescent screens, 3. flat LED computer screens replacing the old fluorescent ones, and 4. White-light LED streetlamps replacing yellow-light sodium vapor lamps. In each case the light emitted is significantly shifted to the blue and even to the ultraviolet. And giant TV and big computer screens pre-empt larger and larger parts of the total field of vision. The result of greater exposure to blue light is inhibition of expression of melatonin, changes in our circadian sleep rhythms, changes in alertness and possible psychological and disease-susceptibility disturbances. These “blue light” effects have received significant research attention recently and have been discussed in the popular press. Some have even opined that lighting has become a public health issue.
Further, with aging, natural human lenses tend to be yellow and less sensitive to blue and UV. Having such lenses may inhibit melatonin suppression and the natural circadian rhythms. How do these factors interact? The purpose of this blog is to review the relevant research and to summarize the major issues and findings to date.
There is much more that meets the eye than what we see.
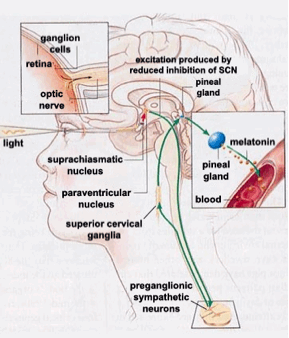
Besides providing visual images, inputs from the eye to other body systems impact on mood, alertness, sense of wellbeing and on the integrity of circadian rhythms related to health. Essentially all vertebrates are subject to a circadian regulatory system where the body adjusts dynamically to the daily cycle of light and dark as well as to how this cycle is affected by season. Key to this process is a small group of hypothalamic nerve cells in the brain, the suprachiasmatic nucleus (SCN), which serves as a master circadian pacemaker. The SCN controls the timing of the sleep-wake cycle and coordinates this with other circadian rhythms to enhance behavioral and environmental adaptation.
“Diagram of various neurological structures, including the suprachiasmatic nucleus (SCN), that are involved in human circadian rhythm control.” Image credit: http://thebrain.mcgill.ca
A starting orientation for this blog is provided by the 2004 publication Human pineal physiology and functional significance of melatonin. “Descriptions of the pineal gland date back to antiquity, but its functions in humans are still poorly understood. In both diurnal and nocturnal vertebrates, its main product, the hormone melatonin, is synthesized and released in rhythmic fashion, during the dark portion of the day-night cycle. Melatonin production is controlled by an endogenous circadian timing system and is also suppressed by light. In lower vertebrates, the pineal gland is photosensitive, and is the site of a self-sustaining circadian clock. In mammals, including humans, the gland has lost direct photosensitivity, but responds to light via a multisynaptic pathway that includes a subset of retinal ganglion cells containing the newly discovered photopigment, melanopsin. The mammalian pineal also shows circadian oscillations, but these damp out within a few days in the absence of input from the primary circadian pacemaker in the suprachiasmatic nuclei (SCN). The duration of the nocturnal melatonin secretory episode increases with nighttime duration, thereby providing an internal calendar that regulates seasonal cycles in reproduction and other functions in photoperiodic species. Although humans are not considered photoperiodic, the occurrence of seasonal affective disorder (SAD) and its successful treatment with light suggest that they have retained some photoperiodic responsiveness. In humans, exogenous melatonin has a soporific effect, but only when administered during the day or early evening, when endogenous levels are low. Some types of primary insomnia have been attributed to diminished melatonin production, particularly in the elderly, but evidence of a causal link is still inconclusive. Melatonin administration also has mild hypothermic and hypotensive effects. A role for the pineal in human reproduction was initially hypothesized on the basis of clinical observations on the effects of pineal tumors on sexual development. More recent data showing an association between endogenous melatonin levels and the onset of puberty, as well as observations of elevated melatonin levels in both men and women with hypogonadism and/or infertility are consistent with such a hypothesis, but a regulatory role of melatonin has yet to be established conclusively. A rapidly expanding literature attests to the involvement of melatonin in immune function, with high levels promoting and low levels suppressing a number of immune system parameters. The detection of melatonin receptors in various lymphoid organs and in lymphocytes suggests multiple mechanisms of action. Melatonin has been shown to be a powerful antioxidant, and has oncostatic properties as well, both direct and indirect, the latter mediated by its effects on reproductive hormones. Finally, there are reports of abnormal daily melatonin profiles in a number of psychiatric and neurological disorders, but the significance of such abnormalities is far from clear.”
The role of melatonin in the process is further explained in the 2006 publication Melatonin: Nature’s most versatile biological signal? “Melatonin is a ubiquitous molecule and widely distributed in nature, with functional activity occurring in unicellular organisms, plants, fungi and animals. In most vertebrates, including humans, melatonin is synthesized primarily in the pineal gland and is regulated by the environmental light/dark cycle via the suprachiasmatic nucleus. Pinealocytes function as ‘neuroendocrine transducers’ to secrete melatonin during the dark phase of the light/dark cycle and, consequently, melatonin is often called the ‘hormone of darkness’. Melatonin is principally secreted at night and is centrally involved in sleep regulation, as well as in a number of other cyclical bodily activities. Melatonin is exclusively involved in signaling the ‘time of day’ and ‘time of year’ (hence considered to help both clock and calendar functions) to all tissues and is thus considered to be the body’s chronological pacemaker or ‘Zeitgeber’. Synthesis of melatonin also occurs in other areas of the body, including the retina, the gastrointestinal tract, skin, bone marrow and in lymphocytes, from which it may influence other physiological functions through paracrine signaling. Melatonin has also been extracted from the seeds and leaves of a number of plants and its concentration in some of this material is several orders of magnitude higher than its night-time plasma value in humans. Melatonin participates in diverse physiological functions. In addition to its timekeeping functions, melatonin is an effective antioxidant which scavenges free radicals and up-regulates several antioxidant enzymes. It also has a strong antiapoptotic signaling function, an effect which it exerts even during ischemia. Melatonin‘s cytoprotective properties have practical implications in the treatment of neurodegenerative diseases. Melatonin also has immune-enhancing and oncostatic properties. Its ‘chronobiotic’ properties have been shown to have value in treating various circadian rhythm sleep disorders, such as jet lag or shift-work sleep disorder. Melatonin acting as an ‘internal sleep facilitator’ promotes sleep, and melatonin‘s sleep-facilitating properties have been found to be useful for treating insomnia symptoms in elderly and depressive patients. A recently introduced melatonin analog, agomelatine, is also efficient for the treatment of major depressive disorder and bipolar affective disorder. Melatonin‘s role as a ‘photoperiodic molecule’ in seasonal reproduction has been established in photoperiodic species, although its regulatory influence in humans remains under investigation. Taken together, this evidence implicates melatonin in a broad range of effects with a significant regulatory influence over many of the body’s physiological functions.”
Image source: http://en.wikipedia.org/wiki/Circadian_rhythm
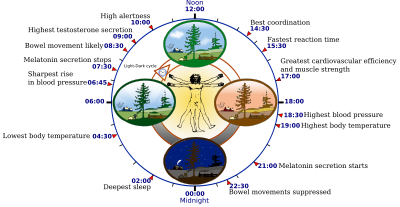
The natural changes in blue light in day and night drives an important aspect of the 24 hour circadian cycle involving the suprachiasmatic nuclei in regulating pineal melatonin synthesis and secretion. In the evenings there is no blue light to inhibit expression of melatonin. Melatonin secretion starts. Melatonin promotes sleep and performs a number of natural antioxidant and other restorative functions(ref)(ref)(ref). Comes daytime, blue light leads to melatonin secretion stopping. Clearing of melatonin resulting in sharper cognitive and memory functions. Artificial blue light at night, possibly generated by LEDs and especially not on a consistent basis, disrupts this cycle. Further, total daily melatonin production may be decreased due to the night work.
There are multiple body clocks. Light at night inhibits melatonin, getting the SCN out of synchronization with the other clocks leading to negative or possibly pathological consequences.
The 2010 publication Circadian dysfunction in disease reports: “The classic view of circadian timing in mammals emphasizes a light-responsive ‘master clock’ within the hypothalamus which imparts temporal information to the organism. Recent work indicates that such a unicentric model of the clock is inadequate. Autonomous circadian timers have now been demonstrated in numerous brain regions and peripheral tissues in which molecular-clock machinery drives rhythmic transcriptional cascades in a tissue-specific manner. Clock genes also participate in reciprocal regulatory feedback with key signalling pathways (including many nuclear hormone receptors), thereby rendering the clock responsive to the internal environment of the body. This implies that circadian-clock genes can directly affect previously unforeseen physiological processes, and that amid such a network of body clocks, internal desynchronisation may be a key aspect to circadian dysfunction in humans. Here we consider the implications of decentralised and internally responsive clockwork to disease, with a focus on energy metabolism and the immune response.”
Quoting from Victor’s recent blog entery Circadian Regulation, NMN, Preventing Diabetes, and Longevity: “Circadian clock functions are ubiquitous and impacted by aging, dietary, lifestyle and environmental conditions. They play important roles with respect to metabolism, health and disease susceptibilities. A key link between circadian regulation and metabolism appears to be the sirtuin SIRT1. Age or disease related-dysregulation of circadian metabolic control can lead to multiple kinds of havoc including type 2 diabetes.”
Blue light seems to be particularly important in driving the daily SCN clock by inhibiting the expression of melatonin.
The fact that the spectral composition of light can impact on the expression of melatonin has been studied since the mod 80s. The effect was discussed in the 1984 publication The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster, the 1985 publication Photoperiodic and light spectral conditions which inhibit circulating concentrations of thyroxine in the male hamster, and the 1986 publication [Effect of UVA on biosynthesis of melatonin in the retina]. From the latter: “Although exposure to UVA light affects the visual process only slightly, melatonin biosynthesis was shown to be influenced significantly. Since melatonin is said to play an important role in light adaptation processes, it may be suggested that an imbalance between visual process and light adaptation may occur, predominantly in the aphakic eye.”
The 2001 publication An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans provided a more detailed report:
- “Using the ability of light to suppress nocturnal melatonin production, we aimed to investigate its spectral sensitivity and produce an action spectrum. Melatonin suppression was quantified in 22 volunteers in 215 light exposure trials using monochromatic light (30 min pulse administered at circadian time (CT) 16-18) of different wavelengths (λmax 424, 456, 472, 496, 520 and 548 nm) and irradiances (0.7-65.0 μW cm−2).
- At each wavelength, suppression of plasma melatonin increased with increasing irradiance. Irradiance-response curves (IRCs) were fitted and the generated half-maximal responses (IR50) were corrected for lens filtering and used to construct an action spectrum.
- The resulting action spectrum showed unique short-wavelength sensitivity very different from the classical scotopic and photopic visual systems. The lack of fit (r2 < 0.1) of our action spectrum with the published rod and cone absorption spectra precluded these photoreceptors from having a major role. Cryptochromes 1 and 2 also had a poor fit to the data. Fitting a series of Dartnall nomograms generated for rhodopsin-based photopigments over the λmax range 420-480 nm showed that rhodopsin templates between λmax 457 and 462 nm fitted the data well (r2≥ 0.73). Of these, the best fit was to the rhodopsin template with λmax 459 nm (r2 = 0.74).
- Our data strongly support a primary role for a novel short-wavelength photopigment in light-induced melatonin suppression and provide the first direct evidence of a non-rod, non-cone photoreceptive system in humans.”
From the 2011 publication Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? “The differential spectral sensitivity of non-image forming responses to visual responses [6], [7] has challenged the classical involvement of rod and cone photopigments in responses to light. Since Berson and co-workers [1] detected intrinsic photosensitive retinal ganglion cell (ipRGC) in the mammalian retina, it began to emerge that the eye plays a dual role in detecting light for a range of behavioral and physiological responses apart from the classical visual responses. Melanopsin-containing ipRGCs have a specialized non-visual retino-hypothalamic tract which provides direct neuronal connections to the suprachiasmatic nucleus (SCN), as well as direct and indirect (via SCN) projections to brain areas implicated in the regulation of arousal [32]. Furthermore, the SCN has connections to the pineal gland, which is responsible for the regulation of melatonin, as well as with many areas that share input from the visual photoreceptor system, such as the lateral geniculate nucleus, pretectum, and superior colliculus [33]. However, very recent findings suggest that cone photoreceptors also contribute substantially to non-visual responses at the beginning of a light exposure and at low irradiances, whereas melanopsin may be the primary circadian photopigment in response to long-duration light exposure and at high irradiances [8].” And yes, it does appear that blue light can keep us alert.
The non-visual impacts of light received by eyes is mediated by retinal ganglion cells which express melanopsin.
These cells are different than the rods and cones which enable us to see objects, and they respond to differently to light spectra, with response peaking in the blue. Discovered in 2000 and as reported in A novel human opsin in the inner retina, “Here we report the of a novel human opsin, melanopsin, that is expressed in cells of the mammalian inner retina. The human melanopsin gene consists of 10 exons and is mapped to chromosome 10q22. This chromosomal localization and gene structure differs significantly from that of other human opsins that typically have four to seven exons. A survey of 26 anatomical sites indicates that, in humans, melanopsin is expressed only in the eye. In situ hybridization histochemistry shows that melanopsin expression is restricted to cells within the ganglion and amacrine cell layers of the primate and murine retinas. Notably, expression is not observed in retinal photoreceptor cells, the opsin-containing cells of the outer retina that initiate vision. The unique inner retinal localization of melanopsin suggests that it is not involved in image formation but rather may mediate nonvisual photoreceptive tasks, such as the regulation of circadian rhythms and the acute suppression of pineal melatonin. The anatomical distribution of melanopsin-positive retinal cells is similar to the pattern of cells known to project from the retina to the suprachiasmatic nuclei of the hypothalamus, a primary circadian pacemaker.”
Also from the 2011 publication Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? “The non-visual effects of ocular light at short wavelengths strongly impinge on the human circadian timing system [1], [2], most probably via novel photoreceptors with the photopigment melanopsin [3], [4], [5]. Maximal response of this non-image-forming (NIF) system to light occurs between 446 and 483 nm for melatonin suppression [6], [7]. Furthermore, circadian phase shifts seem to be more sensitive to 460-nm light compared to 555-nm light at high irradiances [8]. Repercussions on human physiology include increased heart rate and core body temperature after blue (460 nm) but not after green light (550 nm) of equal photon density when administered in the evening [9], together with decreased electroencephalographic (EEG) slow-wave activity in the first cycle of non- rapid eye (NREM) sleep and shortened rapid eye movement (REM) sleep duration in the first two cycles [10].”
Light peaked at 6500K appears to have the highest bioactive effect for melatonin suppression.
The same publication reports “Comparison of salivary melatonin levels across different light conditions indicated that light at 6500K, 3000K and 2500K resulted, respectively, in an increase of 29.5±5%, 49±7.6% and 42±8.6% in comparison to pre-light exposure (1-way ANOVA, F2,17=2.1, p=0.03). –Here we demonstrate that the alerting response to polychromatic light in the evening is wavelength-dependent, such that light at 6500K is more effective than light at 2500K and 3000K in reducing subjective sleepiness and enhancing cognitive performance, specifically associated with tasks of sustained attention. — In our study, light exposure caused a wavelength-dependent suppression of salivary melatonin, such that light at 6500K resulted in a significant attenuated melatonin secretion, particularly after 90 minutes of light exposure, and which persisted during post-light exposure. This stands in agreement with recent findings suggesting that the human circadian pacemaker is highly sensitive to short wavelength light [6], [7], as indexed by action spectra for human melatonin suppression and assessment of human circadian phase resetting [30], [31].”
A trend in street lighting is replacing sodium vapor bulbs with more energy-efficient LED lighting, shifting the spectrum of such light from the yellow toward the blue.
This is happening throughout the country. For example on Feb 22, 2012, the Dothan Eagle reported Energy-efficient street lights being installed reported: “New energy-efficient streetlights are going up on West Main Street, reducing energy consumption and greenhouse gas emissions. — Richard Ash, electric operations supervisor for Dothan Utilities, said the street lights were purchased for the city through a grant from the U.S. Department of Energy, aimed at pursuing energy efficiency to reduce greenhouse gas emissions. The grant was for $600,000. About $100,000 of that is being used for the streetlights. — The new streetlights are 157-watt LED lights and replace 250-watt high pressure sodium bulbs. Ash said the new lights use less electricity and will last longer than the old sodium bulbs. According to Lighting Orient, an LED light manufacturer, LED lights provide 50 to 60 percent energy savings over traditional sodium bulbs. They may also appear brighter than the old bulbs because they emit white, rather than yellow light. — Dothan Utilities workers are currently installing about 68 lights from the intersection of West Main Street at Ross Clark Circle to Montana Street. Lights have also been installed on Westgate Parkway and Honeysuckle Road. Installation should be complete in about two weeks.”
Flat-screen LED TV sets and backlit LED computer monitors have already largely displaced the older fluorescent CRT TV sets and monitors. These new displays often display light whose frequency peak is more in the blue. Blue light from light-emitting diodes at night can cause significant suppression of expression of melatonin in humans with consequent disruption of the daily circadian rhthym with possible negative health consequences.
The May 2011 publication Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance reports: “Many people spend an increasing amount of time in front of computer screens equipped with light-emitting diodes (LED) with a short wavelength (blue range). Thus we investigated the repercussions on melatonin (a marker of the circadian clock), alertness, and cognitive performance levels in 13 young male volunteers under controlled laboratory conditions in a balanced crossover design. A 5-h evening exposure to a white LED-backlit screen with more than twice as much 464 nm light emission {irradiance of 0,241 Watt/(steradian × m(2)) [W/(sr × m(2))], 2.1 × 10(13) photons/(cm(2) × s), in the wavelength range of 454 and 474 nm} than a white non-LED-backlit screen [irradiance of 0,099 W/(sr × m(2)), 0.7 × 10(13) photons/(cm(2) × s), in the wavelength range of 454 and 474 nm] elicited a significant suppression of the evening rise in endogenous melatonin and subjective as well as objective sleepiness, as indexed by a reduced incidence of slow eye movements and EEG low-frequency activity (1-7 Hz) in frontal brain regions. Concomitantly, sustained attention, as determined by the GO/NOGO task; working memory/attention, as assessed by “explicit timing”; and declarative memory performance in a word-learning paradigm were significantly enhanced in the LED-backlit screen compared with the non-LED condition. Screen quality and visual comfort were rated the same in both screen conditions, whereas the non-LED screen tended to be considered brighter. — Our data indicate that the spectral profile of light emitted by computer screens impacts on circadian physiology, alertness, and cognitive performance levels. The challenge will be to design a computer screen with a spectral profile that can be individually programmed to add timed, essential light information to the circadian system in humans.”
So, for people like myself who tend to work at night in front of a large bluish LED screen, compared to working on an old smaller and yellower screen: 1.On the one hand, alertness, cognitive and memory performance and feeling of wellbeing are improved, sleepiness is diminished and productivity is improved, and 2. On the other hand evening expression of melatonin is inhibited, the nighttime circadian sleep cycle is delayed, depth and quality of sleep may be impaired, and there could be additional negative consequences.
The December 2011 publication Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans points out the importance of light frequency on LED inhibition of melatonin. This document reports: “Light suppresses melatonin in humans, with the strongest response occurring in the short-wavelength portion of the spectrum between 446 and 477 nm that appears blue. Blue monochromatic light has also been shown to be more effective than longer-wavelength light for enhancing alertness. Disturbed circadian rhythms and sleep loss have been described as risk factors for astronauts and NASA ground control workers, as well as civilians. Such disturbances can result in impaired alertness and diminished performance. Prior to exposing subjects to short-wavelength light from light-emitting diodes (LEDs) (peak λ = 469 nm; 1/2 peak bandwidth = 26 nm), the ocular safety exposure to the blue LED light was confirmed by an independent hazard analysis using the American Conference of Governmental Industrial Hygienists exposure limits. Subsequently, a fluence-response curve was developed for plasma melatonin suppression in healthy subjects (n = 8; mean age of 23.9 ± 0.5 years) exposed to a range of irradiances of blue LED light. Subjects with freely reactive pupils were exposed to light between 2:00 and 3:30 AM. Blood samples were collected before and after light exposures and quantified for melatonin. The results demonstrate that increasing irradiances of narrowband blue-appearing light can elicit increasing plasma melatonin suppression in healthy subjects (P < 0.0001). The data were fit to a sigmoidal fluence-response curve (R(2) = 0.99; ED(50) = 14.19 μW/cm(2)). A comparison of mean melatonin suppression with 40 μW/cm(2) from 4,000 K broadband white fluorescent light, currently used in most general lighting fixtures, suggests that narrow bandwidth blue LED light may be stronger than 4,000 K white fluorescent light for suppressing melatonin.”
One negative consequence of inhibition of melatonin production by exposure to blue LED light at night could be elevated risk of breast cancer.
The October 2011 publication Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night reports; “This review article discusses recent work on the melatonin-mediated circadian regulation and integration of molecular, dietary, and metabolic signaling mechanisms involved in human breast cancer growth and the consequences of circadian disruption by exposure to light at night (LAN). The antiproliferative effects of the circadian melatonin signal are mediated through a major mechanism involving the activation of MT(1) melatonin receptors expressed in human breast cancer cell lines and xenografts. In estrogen receptor (ERα+) human breast cancer cells, melatonin suppresses both ERα mRNA expression and estrogen-induced transcriptional activity of the ERα via MT(1) -induced activation of G(αi2) signaling and reduction of 3′,5′-cyclic adenosine monophosphate (cAMP) levels. Melatonin also regulates the transactivation of additional members of the steroid hormone/nuclear receptor super-family, enzymes involved in estrogen metabolism, expression/activation of telomerase, and the expression of core clock and clock-related genes. The anti-invasive/anti-metastatic actions of melatonin involve the blockade of p38 phosphorylation and the expression of matrix metalloproteinases. Melatonin also inhibits the growth of human breast cancer xenografts via another critical pathway involving MT(1) -mediated suppression of cAMP leading to blockade of linoleic acid uptake and its metabolism to the mitogenic signaling molecule 13-hydroxyoctadecadienoic acid (13-HODE). Down-regulation of 13-HODE reduces the activation of growth factor pathways supporting cell proliferation and survival. Experimental evidence in rats and humans indicating that LAN-induced circadian disruption of the nocturnal melatonin signal activates human breast cancer growth, metabolism, and signaling provides the strongest mechanistic support, thus far, for population and ecological studies demonstrating elevated breast cancer risk in night shift workers and other individuals increasingly exposed to LAN.”
Photo-triggering of circadian rhythms may be impaired in older people because of yellowing of their natural lenses which blocks blue light.
“The December 2011 publication Short wavelength light filtering by the natural human lens and IOLs – implications for entrainment of circadian rhythm reports: “Purpose: Photoentrainment of circadian rhythm begins with the stimulation of melanopsin containing retinal ganglion cells that respond directly to bluelight. With age, the human lens becomes a strong colour filter attenuating transmission of short wavelengths. — The purpose of the study was to examine the effect the ageing human lens may have for the photoentrainment of circadian rhythm and to compare with intraocular implant lenses (IOLs) designed to block UV radiation, violet or bluelight. Methods: The potential for photoentrainment of circadian rhythm was computed for 29 human donor lenses (18-76 years) and five IOLs (one UV, two violet and two bluelight blocking) based on the transmission properties of the lenses and the spectral characteristics of melanopsin activation and two of it’s physiological outcomes; melanopsin-driven pupillary light reponse and light-induced melatonin suppression. Results: The potential for melanopsin stimulation and melatonin suppression was reduced by 0.6-0.7 percentage point per year of life because of yellowing of the natural lens. The computed effects were small for the IOLs and did not exceed that of a 22.2-year-old natural lens for the blue-blocking IOLs. Conclusion: The results show that photoentrainment of circadian rhythm may be significantly impaired in older subjects because of the colour filtering characteristics of the human lens, whereas the effects were small for all three types of IOLs studied. Consequently, the ageing process of the natural lens is expected to influence the photoentrainment of circadian rhythm, whereas IOLs are not expected to be detrimental to circadian rhythm.”
So, one would think that yellowed lenses would protect older people against LED-originated blue light at night as well as inhibiting light-activated clearance of melatonin in the morning. This is not necessarily the case for people who have had cataract surgery since artificial replacement lenses may readily transmit bluish light. I have had cataract surgery and have an implanted replacement lense only in my left eye. When I look at things with only one eye open, scenes and objects are brighter and bluish seen from the left eye, darker and yellowish when seen from the right eye.
Research has been conducted on intraocular implants with different spectral characteristics. Some are blue-blocking. See these publications. Some researchers feel there are detriments but no health benefits to blue blocking. See the 2010 publication Blue-blocking IOLs decrease photoreception without providing significant photoprotection.
On a personal note
Relevant to the above, typically: 1. I work until 9-10:30 PM on a 26” backlit LED computer monitor or watch TV on a 47” flat LED screen, 2. I take 2mg of melatonin together with other supplements about 10:45PM, 3. I go to bed at 11:30PM, 4. I have no trouble going to sleep or sleeping, 5. When I get up at 7:30 or 8:00 AM, I am often still quite sleepy. Exposing myself to bright light and exercising help shake this off as well as does going back to work in front of the LED monitor. It seems my melatonin supplement has a 10-hour impact.


I am investigating “live fast die young”.
A star is born?
Pingback: Quora
I use amber glasses designed to filter out blue light and initiate the pineal glands response to release melatonin. I put them on about an hour before hitting the sack. The glasses work for me, I find theme to be very effective.
Pingback: Lighting for Health Blue light and sleep, mental alertness and health - Lighting for Health
Wellness:
What an interesting and simple idea. Put sunglasses on an hour before bed if watching TV or using a computer.
Commuicating electronic devices that measure personal light exposure may help too. It has just come to my attention that GoodLux Technology recently launched SunSprite, the first wearable device to track daily bright light intake.
Vince
Pingback: Digital health – health and fitness wearables, apps and platforms – implications for assessing health and longevity interventions – Part 1 Flux in the market | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Homepage