By Vince Giuliano
To take care of your own health you have to take care of about a hundred trillion other living entities. Each of us is in close relationship with that many micro-organisms belonging to hundreds of different species that live in our intestines. Our own health and longevity is closely tied up to the balance among these species and their vitality. Recent research indicates that signals sent out by colon microbiota directly affect our immune systems, states of health, and can either avert or lead to and promote disease conditions. It is thought that new and effective therapies for many disease conditions might be based on working with gut microbiota. And new research shows that promoting an optimal balance of gut microbiota species in mice can extend their lifespans. Probiotics and prebiotics could be life-saving and life-prolonging.
There is strong and widespread current interest in gut microbiota and I have found thousands of relevant research publications related to gut microbiota. My purpose here it to introduce the topic in this blog, to establish its relevance, and to identify some recent key research findings.
Introduction
Aging of a human happens in a meta-organism consisting not only of that person’s body and cells but also of the 100 trillion organisms that exist in that persons gut.
This is a mind-numbing proposition, as if aging were not complicated enough just considering the person. To introduce the topic I quote from the just-published (Feb 2012) article Ageing of the human metaorganism: the microbial counterpart: “Human beings have been recently reviewed as ‘metaorganisms’ as a result of a close symbiotic relationship with the intestinal microbiota. This assumption imposes a more holistic view of the ageing process where dynamics of the interaction between environment, intestinal microbiota and host must be taken into consideration. — Age-related physiological changes in the gastrointestinal tract, as well as modification in lifestyle, nutritional behaviour, and functionality of the host immune system, inevitably affect the gut microbial ecosystem. Here we review the current knowledge of the changes occurring in the gut microbiota of old people, especially in the light of the most recent applications of the modern molecular characterisation techniques. The hypothetical involvement of the age-related gut microbiota unbalances in the inflamm-aging, and immunosenescence processes will also be discussed. Increasing evidence of the importance of the gut microbiota homeostasis for the host health has led to the consideration of medical/nutritional applications of this knowledge through the development of probiotic and prebiotic preparations specific for the aged population. The results of the few intervention trials reporting the use of pro/prebiotics in clinical conditions typical of the elderly will be critically reviewed.”
Image from customprobiotics.com
The typical mix of microbiota in the gut changes from infancy to adulthood, remains fairly stable, and then changes again in old age.
“Every time a human baby is born, a rich and dynamic microbial ecosystem develops from a sterile environment. During the first year of life, until weaning, the gut ecosystem is prevalently colonized by opportunistic microorganisms to which a baby is exposed in its environment (Palmer et al. 2007). The earliest colonizers are often aerobes such as Staphylococcus, Streptococcus and Enterobacteria, followed by anaerobic later colonizers such as Eubacteria and Clostridia. After these earlier stages, it is generally though that the microbiota of breast-fed infants is largely dominated by Bifidobacterium. After weaning, the developmental changes in the gut mucosa and in the intestinal IS, together the introduction of a solid diet, drive to the transition to a resilience adult-like profile of the human gut microbiota, characterised by a remarkable microbial biodiversity. The ageing of the gut microbiota starts after a subject-specific ‘threshold age’ which depends on individual characteristics such as diet, country and, eventually, frailty. In any case, changes of diet, lifestyle and the immunosenescence of the intestinal IS dramatically impact the microbial ecology of the human GI tract. Symmetrically to what happens in the early stage of our life, the aged-type microbiota shows a low microbial biodiversity, and it is characterised by an increase in opportunistic environmental facultative aerobes Staphylococcus, Streptococcus, Enterobacteriaceae, as well as a reduction in anaerobes, such as Clostridium clusters IV and XIVa and Bacteroidetes. However, differently from the infant-type microbiota, the aged type is characterised by a low abundance of Bifidobacterium(ref).”
The 2009 publication Acquisition, evolution and maintenance of the normal gut microbiota reports: “The gut is sterile at birth, but is rapidly colonised by faecal and vaginal bacteria of maternal origin. Over the succeeding weeks, months and years, a complex microbiota develops that plays a major role in host physiology. While the digestive tract is colonised to varying degrees by micro-organisms throughout its length, due to acid pH and the short retention time of gastric contents, bacterial numbers in the stomach are usually low. The rapid passage of digestive materials through the upper gut does not provide time for significant bacterial growth to occur, but cell numbers increase considerably in the distal ileum. The rate of movement of intestinal contents slows in the colon, which facilitates the development of complex bacterial communities. The large intestine is an intricate ecosystem that contains a complex microbiota composed of several hundred different types of bacteria. The growth and metabolism of microbial communities in the large intestine are determined by many factors, such as diet, environment and host physiological processes, as well as the anatomic structure of the digestive tract, disease, immunity, host genetics, drugs and ageing. Modifications in diet and host immune system activity, as well as physiological changes in the digestive tract affect microbiota composition in older people. The elderly have fewer bifidobacteria and higher numbers of enterobacteria and clostridia than young adults. Increased antibiotic use in older people and simply going into hospital have been shown to change bacterial community structure in the colonic microbiota, although the metabolic significance of this is unclear.”
From Genomic Insights into Bifidobacteria: “The human large intestine is a very complex ecosystem that is still not fully understood, and while its microbial composition consists primarily of just four bacterial phyla, Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria (as well as some Archaea, Eukarya, and viruses), it is highly variable at the genus level between individuals, likely due to factors such as age, health, diet, etc. (25, 99, 127, 244). Therefore, the adaptation capabilities of the intestinal bacteria corresponding to these variable factors likely influence the overall composition of intestinal microflora in the intestine (89).”
The 2007 publication Intestinal bacteria and ageing reported: “Advancements in science and medicine, as well as improved living standards, have led to a steady increase in life expectancy, and subsequently a rise in the elderly population. The intestinal microbiota is important for maintenance of host health, providing energy, nutrients and protection against invading organisms. Although the colonic microbiota is relatively stable throughout adult life, age-related changes in the gastrointestinal (GI) tract, as well as changes in diet and host immune system reactivity, inevitably affect population composition. Recent studies indicate shifts in the composition of the intestinal microbiota, which may lead to detrimental effects for the elderly host. Increased numbers of facultative anaerobes, in conjunction with a decrease in beneficial organisms such as the anaerobic lactobacilli and bifidobacteria, amongst other anaerobes, have been reported. These changes, along with a general reduction in species diversity in most bacterial groups, and changes to diet and digestive physiology such as intestinal transit time, may result in increased putrefaction in the colon and a greater susceptibility to disease. — Therapeutic strategies to counteract these changes have been suggested in ageing people. These include dietary supplements containing prebiotics, probiotics and a combination of both of these, synbiotics. Limited feeding trials show promising results with these supplements, although further longer-term investigations are required to substantiate their use in elderly healthcare fields.”
The 2010 publication Role of the gut microbiota in defining human health reported: “The human superorganism is a conglomerate of mammalian and microbial cells, with the latter estimated to outnumber the former by ten to one and the microbial genetic repertoire (microbiome) to be approximately 100-times greater than that of the human host. Given the ability of the immune response to rapidly counter infectious agents, it is striking that such a large density of microbes can exist in a state of synergy within the human host. This is particularly true of the distal gastrointestinal (GI) tract, which houses up to 1000 distinct bacterial species and an estimated excess of 1 x 10(14) microorganisms. An ever-increasing body of evidence implicates the GI microbiota in defining states of health and disease. Here, we review the literature in adult and pediatric GI microbiome studies, the emerging links between microbial community structure, function, infection and disease, and the approaches to manipulate this crucial ecosystem to improve host health.”
While the study of gut bacteria has been going on for more than 100 years, many new insights are now flowing from massive genomic sequencing techniques.
Bifidobacteria appear to be an important set of species of gut bacteria from a health viewpoint.
Study of the relationships of gut bacteria to health are very far from new. Identification of Bifidobacteria goes back to 1900. Also from the September 2010 publication Genomic Insights into Bifidobacteria “Tissier (315) suggested that the large number of bifidobacteria in the feces of healthy breast-fed infants was likely the reason for their lower incidence of infantile diarrhea. In his pediatric work, he used bifidobacteria for the treatment of this intestinal diarrhea, and this likely represents the first example of the oral administration of a live microorganism for the treatment of a disease (316). The abundance of bifidobacteria in the feces of breast-fed infants was thought to be due to the Bifidobacterium-stimulating properties of human breast milk (38, 46, 60, 96, 196, 321). Numerous studies have substantiated the higher bifidobacterial counts and lower incidences of gastroenteritis in breast-fed infants than in formula-fed infants (3, 40, 51, 98).” The Summary of the publication relates: “Since the discovery in 1899 of bifidobacteria as numerically dominant microbes in the feces of breast-fed infants, there have been numerous studies addressing their role in modulating gut microflora as well as their other potential health benefits. Because of this, they are frequently incorporated into foods as probiotic cultures. An understanding of their full interactions with intestinal microbes and the host is needed to scientifically validate any health benefits they may afford. Recently, the genome sequences of nine strains representing four species of Bifidobacterium became available. A comparative genome analysis of these genomes reveals a likely efficient capacity to adapt to their habitats, with B. longum subsp. infantis exhibiting more genomic potential to utilize human milk oligosaccharides, consistent with its habitat in the infant gut. Conversely, B. longum subsp. longum exhibits a higher genomic potential for utilization of plant-derived complex carbohydrates and polyols, consistent with its habitat in an adult gut. An intriguing observation is the loss of much of this genome potential when strains are adapted to pure culture environments, as highlighted by the genomes of B. animalis subsp. lactis strains, which exhibit the least potential for a gut habitat and are believed to have evolved from the B. animalis species during adaptation to dairy fermentation environments.”
“The composition of the microflora in the human large intestine, as estimated using culturing techniques, is usually dominated by the genera Bacteroides, Eubacterium, and Bifidobacterium, with several other predominant genera, such as Clostridium, Peptostreptococcus, Enterococcus, Lactobacillus, and members of the family Enterobacteriaceae (117, 118). The number of species estimated by culturing techniques is approximately 400. Nonculturing analysis of the gut microflora was greatly facilitated by the direct isolation of DNA from feces and by amplification of the 16S rRNA genes representing the entire microflora. Cloning and sequencing of individual rRNA genes enabled the numerically dominant genera of bacteria to be identified. This molecular analysis of the intestinal microflora in fecal and colonic samples initially suggested that the human large intestine contains more than 500 different bacterial species and that about 75% of them are nonculturable (81, 83). However, a recent extensive metagenomic analysis revealed that this is an overestimation, with most individuals harboring approximately 160 different bacterial species, the majority of which are “known” (239). While the total number of species present in the human gut is not known, recent molecular studies indicate that it is in excess of 1,000 (239, 241), with an upper estimate of 1,150 suggested by an extensive metagenomic analysis of 124 individuals (239)(ref).”
Gut microflora may play an even more important role in maintaining human health than previously thought. Specifically it appears they are involved in the control of energy and metabolic homeostasis.
This theme has been repeated in a substantial number of publications for over five years now. The 2007 reviewpublication Gut microflora as a target for energy and metabolic homeostasis reported “Purpose of review: Gut microbiota plays an important role in health and disease, but this ecosystem remains incompletely characterized and shows a wide diversity. This review discusses new findings that may explain how gut microbiota can be involved in the control of energy and metabolic homeostasis. Recent findings: Over the past 5 years studies have highlighted some key aspects of the mammalian host-gut microbial relationship. Gut microbiota could now be considered a ‘microbial organ’ placed within a host organ. Recent data suggest that the modulation of gut microbiota affects host metabolism and has an impact on energy storage. Several mechanisms are proposed that link events occurring in the colon and the regulation of energy metabolism. Summary: Gut microflora may play an even more important role in maintaining human health than previously thought. The literature provides new evidence that the increased prevalence of obesity and type 2 diabetes cannot be attributed solely to changes in the human genome, nutritional habits, or reduction of physical activity in our daily lives. One must also consider this important new environmental factor, namely gut microbiota. Scientists may take into consideration a key question: could we manipulate the microbiotic environment to treat or prevent obesity and type 2 diabetes? This opens up a new area in nutrition research.”
The August 2011 publication Programming of host metabolism by the gut microbiota reports: “The human gut harbors a vast ensemble of bacteria that has co-evolved with the human host and performs several important functions that affect our physiology and metabolism. The human gut is sterile at birth and is subsequently colonized with bacteria from the mother and the environment. The complexity of the gut microbiota is increased during childhood, and adult humans contain 150-fold more bacterial genes than human genes. Recent advances in next-generation sequencing technology and mechanistic testing in gnotobiotic mice have identified the gut microbiota as an environmental factor that contributes to obesity. Germ-free mice are protected against developing diet-induced obesity and the underlying mechanisms whereby the gut microbiota contributes to host metabolism are beginning to be clarified. The obese phenotype is associated with increased microbial fermentation and energy extraction; however, other microbially modulated mechanisms contribute to disease progression as well. The gut microbiota has profound effects on host gene expression in the enterohepatic system, including genes involved in immunity and metabolism. For example, the gut microbiota affects expression of secreted proteins in the gut, which modulate lipid metabolism in peripheral organs. In addition, the gut microbiota is also a source of proinflammatory molecules that augment adipose inflammation and macrophage recruitment by signaling through the innate immune system. TLRs (Toll-like receptors) are integral parts of the innate immune system and are expressed by both macrophages and epithelial cells. Activation of TLRs in macrophages dramatically impairs glucose homeostasis, whereas TLRs in the gut may alter the gut microbial composition that may have profound effects on host metabolism. Accordingly, reprogramming the gut microbiota, or its function, in early life may have beneficial effects on host metabolism later in life.”
The mixture of kinds of bacteria in the gut may contribute to metabolic disorders such as obesity, diabetes, and cardiovascular diseases or can contribute strongly to maintenance of health homeostasis.
The November 2010 publication Ecology and Physiology of the Intestinal Tract reports: “The number of microorganisms inhabiting the human digestive tract exceeds the number of body cells by a factor of ten. This microbial community affects host physiology and host health. The metabolic potential of the gut microbiota is immense affording the extraction of energy from otherwise indigestible carbohydrates (dietary fiber) and the conversion of host-derived substances, non-nutritive dietary components and drugs. Recognized functions of the gut microbiota include provision of colonization resistance against pathogens and priming of both the innate and the acquired immune systems. However, the intestinal microbiota may also contribute to the development of diseases such as ulcerative colitis and colorectal cancer. Culture-dependent studies provided basic knowledge on the gut microbiota, but only the advent of culture-independent molecular methods led to a better understanding of host-microbe interactions. The application of metagenomics to the gut microbial ecosystem revealed truly remarkable correlations between certain diseases and the gut microbiome. It also led to the suggestion of the existence of a ‘core microbiome’ that encompasses key functions shared by each individual. However, the mechanisms underlying host-microbe interactions have not yet been unraveled.”” 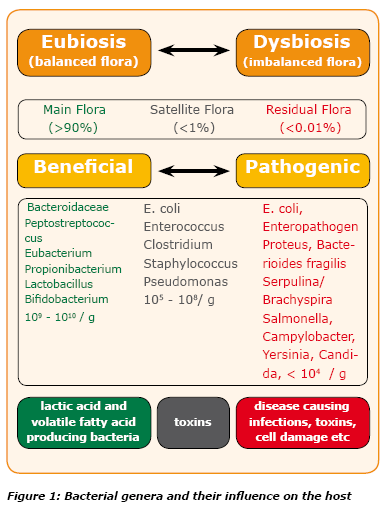
Image from website of engormix.com.
The January 2012 publication Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? Relates: “The gut microbiota refers to the trillions of microorganisms residing in the intestine and is integral in multiple physiological processes of the host. Recent research has shown that gut bacteria play a role in metabolic disorders such as obesity, diabetes, and cardiovascular diseases. The mechanisms by which the gut microbiota affects metabolic diseases are by two major routes: (1) the innate immune response to the structural components of bacteria (e.g., lipopolysaccharide) resulting in inflammation and (2) bacterial metabolites of dietary compounds (e.g., SCFA from fiber), which have biological activities that regulate host functions. Gut microbiota has evolved with humans as a mutualistic partner, but dysbiosis in a form of altered gut metagenome and collected microbial activities, in combination with classic genetic and environmental factors, may promote the development of metabolic disorders. This paper reviews the available literature about the gut microbiota and aforementioned metabolic disorders and reveals the gaps in knowledge for future study.”
Other relevant publications include:
The environment within: how gut microbiota may influence metabolism and body composition (2010)
A discussion of the role of gut flora in autism and inflammatory bowel disease can be found on this webpage of science-autism.org which is about published scientific data concerning autism. The following diagram is also from that web page. It illustrates the role microbiota can play in breaching the intestinal barrier wall and generating an inflammatory reaction.
Image from Gut Flora in Autism: “A well considered pathway for the effect of bacterial flora on the inflammatory response of the gut wall in IBD. The inflammation response starts when the gut barrier is breeched and this causes a further inflammatory response.”
With advanced aging, changes typically happen in the ecology of the gut microorganism, system resulting in increased susceptibility to infectious diseases and related infirmities.
The 2010 publication Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians reports: “Age-related physiological changes in the gastrointestinal tract, as well as modifications in lifestyle, nutritional behaviour, and functionality of the host immune system, inevitably affect the gut microbiota, resulting in a greater susceptibility to infections. — Methodology/Principal Findings: By using the Human Intestinal Tract Chip (HITChip) and quantitative PCR of 16S rRNA genes of Bacteria and Archaea, we explored the age-related differences in the gut microbiota composition among young adults, elderly, and centenarians, i.e subjects who reached the extreme limits of the human lifespan, living for over 100 years. We observed that the microbial composition and diversity of the gut ecosystem of young adults and seventy-years old people is highly similar but differs significantly from that of the centenarians. After 100 years of symbiotic association with the human host, the microbiota is characterized by a rearrangement in the Firmicutes population and an enrichment in facultative anaerobes, notably pathobionts. The presence of such a compromised microbiota in the centenarians is associated with an increased inflammatory status, also known as inflammageing, as determined by a range of peripheral blood inflammatory markers. This may be explained by a remodelling of the centenarians’ microbiota, with a marked decrease in Faecalibacterium prauznitzii and relatives, symbiotic species with reported anti-inflammatory properties. As signature bacteria of the long life we identified specifically Eubacterium limosum and relatives that were more than ten-fold increased in the centenarians. Conclusions/Significance: We provide evidence for the fact that the ageing process deeply affects the structure of the human gut microbiota, as well as its homeostasis with the host’s immune system. Because of its crucial role in the host physiology and health status, age-related differences in the gut microbiota composition may be related to the progression of diseases and frailty in the elderly population.”
Image from the website of Les Laboratoires Nicar, Inc.
On the other hand, the 2010 e-publication Human intestinal microbiota and healthy ageing review publication reports: “Earlier studies have indicated a decrease in anaerobes and bifidobacteria and a concomitant increase in enterobacteria in the intestinal microbiota with ageing. However, new data obtained with molecular techniques suggests decreased stability and increased diversity of the gut microbiota with advancing age. Further, no simple marker change in microbiota composition can be identified. Except for the reduced immune function, ageing itself may have relatively little effect on overall gastrointestinal function. Concomitant changes in nutrition, increased incidence of disease and corresponding use of medication with advancing age modify the composition of the microbial community of the gastrointestinal tract. This mini-review will focus on the recent findings on the gut microbiota of the elderly and on the potential benefits of probiotics, prebiotics and synbiotics.”
TLR and NLR signaling in intestinal epithelial cells triggered by gut microbiota contribute to a range of homeostatic mechanisms including proliferation, wound healing, epithelial integrity, and regulation of mucosal immune functions.
The 2010 publication The role of innate signaling in the homeostasis of tolerance and immunity in the intestine reports: “In the intestine innate recognition of microbes is achieved through pattern recognition receptor (PRR) families expressed in immune cells and different cell lineages of the intestinal epithelium. Toll-like receptor (TLR) and nucleotide-binding and oligomerization domain-like receptor (NLR) families are emerging as key mediators of immunity through their role as maturation factors of immune cells and triggers for the production of cytokines and chemokines and antimicrobial factors. At the mucosal surface chronic activation of the immune system is avoided through the epithelial production of a glycocalyx, steady-state production of antimicrobial factors as well as the selective expression and localization of PRRs. Additionally, the polarization of epithelial TLR signaling and suppression of NF-kappaB activation by luminal commensals appears to contribute to the homeostasis of tolerance and immunity. Several studies have demonstrated that TLR signaling in epithelial cells contributes to a range of homeostatic mechanisms including proliferation, wound healing, epithelial integrity, and regulation of mucosal immune functions. The intestinal epithelium appears to have uniquely evolved to maintain mucosal tolerance and immunity, and future efforts to further understand the molecular mechanisms of intestinal homeostasis may have a major impact on human health.”
Our old friend NF-kappaB can be activated by TLR and NOD signaling by gut microbiota, resulting in possible positive or detrimental effects.
The 2010 publication Role of NF-kappaB activation in intestinal immune homeostasis reports: “Inflammatory bowel diseases (IBD) are characterised by a disturbance of intestinal immune homeostasis, either caused by or followed by inappropriate responses to the resident commensal bacteria. Although the transcription factor NF-kappaB actively participates in the excessive inflammatory response observed in IBD, recent studies with mice defective in NF-kappaB activation have revealed that NF-kappaB also serves an essential protective function in the intestinal immune system. The enormous amount of commensal bacteria in the intestine might play a role in the distinct functions of NF-kappaB in the intestine, as they can initiate signalling to NF-kappaB through both Toll-like receptors and NOD-like receptors in intestinal epithelial cells as well as mucosal immune cells. However, the exact individual contributions of different NF-kappaB-activating stimuli as well as the target cells that mediate the detrimental or beneficial functions of NF-kappaB in the intestine are still elusive. In this review, I will summarise and discuss the current knowledge on the role of different NF-kappaB-activating pathways in preserving intestinal immune homeostasis and the development of intestinal inflammation.”
Gut microbiota have a strong impact on immune system responses.
The May 2011 publication Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma reports: “The human intestine contains more than 100 trillion microorganisms that maintain a symbiotic relationship with the host. Under normal conditions, these bacteria are not pathogenic and in fact confer health benefits to the host. The microbiota interacts with the innate and adaptive arms of the host’s intestinal mucosal immune system and through these mechanisms drives regulatory cell differentiation in the gut that is critically involved in maintaining immune tolerance. Specifically, the microbiota can activate distinct tolerogenic dendritic cells in the gut and through this interaction can drive regulatory T-cell differentiation. In addition, the microbiota is important in driving T(H)1 cell differentiation, which corrects the T(H)2 immune skewing that is thought to occur at birth. If appropriate immune tolerance is not established in early life and maintained throughout life, this represents a risk factor for the development of inflammatory, autoimmune, and allergic diseases. Early-life events are instrumental in establishing the microbiota, the composition of which throughout life is influenced by various environmental and lifestyle pressures. Significant efforts are now being made to establish interventional approaches that can create a healthy microbiota that confers maximum tolerogenic immunomodulatory effects in the gut and that will protect against systemic inflammatory disease pathologies.”
Regarding probiotics, prebiotics and synbiotics
Practical interventions in gut bacteria mixes are primarily based on taking probiotic, prebiotic or symbiotic supplements. In a nutshell, a probiotic supplement contains one or more strains of live bacteria known to be health-promoting. A prebiotic is a dietary supplement designed to promote the proliferation and sustainability in the gut of health-producing bacterial strains. And a symbiotic is blend of a probiotic and a probiotic. The distinctions are discussed in the 2008 publication Probiotics, prebiotics, and synbiotics: “.According to the German definition, probiotics are defined viable microorganisms, sufficient amounts of which reach the intestine in an active state and thus exert positive health effects. Numerous probiotic microorganisms (e.g. Lactobacillus rhamnosus GG, L. reuteri, bifidobacteria and certain strains of L. casei or the L. acidophilus-group) are used in probiotic food, particularly fermented milk products, or have been investigated–as well as Escherichia coli strain Nissle 1917, certain enterococci (Enterococcus faecium SF68) and the probiotic yeast Saccharomyces boulardii–with regard to their medicinal use. Among the numerous purported health benefits attributed to probiotic bacteria, the (transient) modulation of the intestinal microflora of the host and the capacity to interact with the immune system directly or mediated by the autochthonous microflora, are basic mechanisms. They are supported by an increasing number of in vitro and in vivo experiments using conventional and molecular biologic methods. In addition to these, a limited number of randomized, well-controlled human intervention trials have been reported. Well-established probiotic effects are: 1. Prevention and/or reduction of duration and complaints of rotavirus-induced or antibiotic-associated diarrhea as well as alleviation of complaints due to lactose intolerance. 2. Reduction of the concentration of cancer-promoting enzymes and/or putrefactive (bacterial) metabolites in the gut. 3. Prevention and alleviation of unspecific and irregular complaints of the gastrointestinal tracts in healthy people. 4. Beneficial effects on microbial aberrancies, inflammation and other complaints in connection with: inflammatory diseases of the gastrointestinal tract, Helicobacter pylori infection or bacterial overgrowth. 5. Normalization of passing stool and stool consistency in subjects suffering from obstipation or an irritable colon. 6. Prevention or alleviation of allergies and atopic diseases in infants. 7. Prevention of respiratory tract infections (common cold, influenza) and other infectious diseases as well as treatment of urogenital infections. Insufficient or at most preliminary evidence exists with respect to cancer prevention, a so-called hypocholesterolemic effect, improvement of the mouth flora and caries prevention or prevention or therapy of ischemic heart diseases or amelioration of autoimmune diseases (e.g. arthritis). A prebiotic is “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well being and health”, whereas synergistic combinations of pro- and prebiotics are called synbiotics. Today, only bifidogenic, non-digestible oligosaccharides (particularly inulin, its hydrolysis product oligofructose, and (trans)galactooligosaccharides), fulfill all the criteria for prebiotic classification. They are dietary fibers with a well-established positive impact on the intestinal microflora. Other health effects of prebiotics (prevention of diarrhoea or obstipation, modulation of the metabolism of the intestinal flora, cancer prevention, positive effects on lipid metabolism, stimulation of mineral adsorption and immunomodulatory properties) are indirect, i.e. mediated by the intestinal microflora, and therefore less-well proven. In the last years, successful attempts have been reported to make infant formula more breast milk-like by the addition of fructo- and (primarily) galactooligosaccharides.”
The 2010 publication Prebiotic effects: metabolic and health benefits relates general experience with probiotics over a 15 year time frame: “The different compartments of the gastrointestinal tract are inhabited by populations of micro-organisms. By far the most important predominant populations are in the colon where a true symbiosis with the host exists that is a key for well-being and health. For such a microbiota, ‘normobiosis’ characterises a composition of the gut ‘ecosystem’ in which micro-organisms with potential health benefits predominate in number over potentially harmful ones, in contrast to ‘dysbiosis’, in which one or a few potentially harmful micro-organisms are dominant, thus creating a disease-prone situation. The present document has been written by a group of both academic and industry experts (in the ILSI Europe Prebiotic Expert Group and Prebiotic Task Force, respectively). It does not aim to propose a new definition of a prebiotic nor to identify which food products are classified as prebiotic but rather to validate and expand the original idea of the prebiotic concept (that can be translated in ‘prebiotic effects’), defined as: ‘The selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host.’ Thanks to the methodological and fundamental research of microbiologists, immense progress has very recently been made in our understanding of the gut microbiota. A large number of human intervention studies have been performed that have demonstrated that dietary consumption of certain food products can result in statistically significant changes in the composition of the gut microbiota in line with the prebiotic concept. Thus the prebiotic effect is now a well-established scientific fact. The more data are accumulating, the more it will be recognised that such changes in the microbiota’s composition, especially increase in bifidobacteria, can be regarded as a marker of intestinal health. The review is divided in chapters that cover the major areas of nutrition research where a prebiotic effect has tentatively been investigated for potential health benefits. The prebiotic effect has been shown to associate with modulation of biomarkers and activity(ies) of the immune system. — Confirming the studies in adults, it has been demonstrated that, in infant nutrition, the prebiotic effect includes a significant change of gut microbiota composition, especially an increase of faecal concentrations of bifidobacteria. This concomitantly improves stool quality (pH, SCFA, frequency and consistency), reduces the risk of gastroenteritis and infections, improves general well-being and reduces the incidence of allergic symptoms such as atopic eczema. Changes in the gut microbiota composition are classically considered as one of the many factors involved in the pathogenesis of either inflammatory bowel disease or irritable bowel syndrome. — The use of particular food products with a prebiotic effect has thus been tested in clinical trials with the objective to improve the clinical activity and well-being of patients with such disorders. Promising beneficial effects have been demonstrated in some preliminary studies, including changes in gut microbiota composition (especially increase in bifidobacteria concentration). Often associated with toxic load and/or miscellaneous risk factors, colon cancer is another pathology for which a possible role of gut microbiota composition has been hypothesised. — Numerous experimental studies have reported reduction in incidence of tumours and cancers after feeding specific food products with a prebiotic effect. Some of these studies (including one human trial) have also reported that, in such conditions, gut microbiota composition was modified (especially due to increased concentration of bifidobacteria). Dietary intake of particular food products with a prebiotic effect has been shown, especially in adolescents, but also tentatively in postmenopausal women, to increase Ca absorption as well as bone Ca accretion and bone mineral density. Recent data, both from experimental models and from human studies, support the beneficial effects of particular food products with prebiotic properties on energy homaeostasis, satiety regulation and body weight gain. Together, with data in obese animals and patients, these studies support the hypothesis that gut microbiota composition (especially the number of bifidobacteria) may contribute to modulate metabolic processes associated with syndrome X, especially obesity and diabetes type 2. It is plausible, even though not exclusive, that these effects are linked to the microbiota-induced changes and it is feasible to conclude that their mechanisms fit into the prebiotic effect. However, the role of such changes in these health benefits remains to be definitively proven. As a result of the research activity that followed the publication of the prebiotic concept 15 years ago, it has become clear that products that cause a selective modification in the gut microbiota’s composition and/or activity(ies) and thus strengthens normobiosis could either induce beneficial physiological effects in the colon and also in extra-intestinal compartments or contribute towards reducing the risk of dysbiosis and associated intestinal and systemic pathologies.”
Raising bifidobacteria levels by the use of probiotics and prebiotics appears to be the most-established strategy for modifying microbial balance in human colons to promote health. However, much remains to be learned.
The December 2009 publication Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects reports: “The most well known food-based strategies to modulate the composition of the intestinal microbiota are the dietary use of prebiotics, probiotics and their combination, synbiotics. Currently established prebiotic compounds are mainly targeting the bifidobacteria population of the colon microbiota. A good illustration of the importance of high colonic bifidobacteria levels is the observation that breast milk creates an environment in the colon (because of its high amount in galacto-oligosaccharides with prebiotic activity) favouring the development of a simple flora, dominated by bifidobacteria to which various health benefits have been ascribed. — Currently, high colonic bifidobacteria levels has been considered favourably at all ages and strategies to augment their presence have been demonstrated in placebo-controlled intervention studies; e.g. in toddlers to reduce sickness events, in adults to reduce the risk for developing gastrointestinal diseases and in the elderly to re-enhance their declining immune activity. The intestinal microbiota can be considered as a metabolically adaptable and rapidly renewable organ of the body. However, unbalances in its microbial community and activities are found to be implicated in disease initiation and progression, such as chronic inflammatory bowel diseases and colonic cancers. Restoration of this balance by increasing bifidobacteria levels has demonstrated to reduce disease severity of patients and to improve well-being in healtly volunteers. New emerging evidence on the difference in the composition of the colonic microbiota between obese and lean volunteers has opened new areas for pre-, pro- and synbiotic research. Additionally, as knowledge will increase about the microbial bio-conversion of polyphenolic compounds into bioactive metabolites in the colon and whether food-based strategies can augment such bioconversion into more potent compounds with anti-oxidant and/or anti-inflammatory activity new areas of research will be discovered. This paper provides an up-to-date review of the health benefits associated to the induction of high bifidobacteria levels in the colon by the use of prebiotics (inulin and oligofructose).”
Prebiotics may be useful for the prevention or treatment of insulin resistance, diabetes and obesity.
The 2011 publication Gut microbiota and the pathogenesis of insulin resistance reports: “Several reviews recently explored how the gut microbiota was able to control host energy metabolism, and thereby the development of adiposity. In this review, we focused on the state of the art that supports a link between the gut microbiota composition and activity, and the management of glycemia associated with overweight and diabetes. Several microbial-derived compounds are related to disturbances of glucose homeostasis including the gram-negative-derived lipopolysaccharides. Some nutrients with prebiotic properties, which escape the digestion in the upper part of the gut, modify the composition of the gut microbiota in favor of bacteria that could play a beneficial role on glucose homeostasis, namely by modulating the endocrine function of the gut, and by reinforcing the gut barrier. Adequate intervention studies in diabetic patients are required to assess the relevance of those experimental data for human health.”
The August 2011 publication Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome reports: “The gut microbiota is increasingly considered as a symbiotic partner for the maintenance of health. The homeostasis of the gut microbiota is dependent on host characteristics (age, gender, genetic background…), environmental conditions (stress, drugs, gastrointestinal surgery, infectious and toxic agents…). Moreover, it is dependent on the day-to-day dietary changes. — Experimental data in animals, but also observational studies in obese patients, suggest that the composition of the gut microbiota is a factor characterizing obese versus lean individuals, diabetic versus non diabetic patients, or patients presenting hepatic diseases such as non alcoholic steatohepatitis. Interestingly, the changes in the gut microbes can be reversed by dieting and related weight loss. The qualitative and quantitative changes in the intake of specific food components (fatty acids, carbohydrates, micronutrients, prebiotics, probiotics), have not only consequences on the gut microbiota composition, but may modulate the expression of genes in host tissues such as the liver, adipose tissue, intestine, muscle. This in turn may drive or lessen the development of fat mass and metabolic disturbances associated with the gut barrier function and the systemic immunity. The relevance of the prebiotic or probiotic approaches in the management of obesity in humans is supported by few intervention studies in humans up to now, but the experimental data obtained with those compounds help to elucidate novel potential molecular targets relating diet with gut microbes. The metagenomic and integrative metabolomic approaches could help elucidate which bacteria, among the trillions in human gut, or more specifically which activities/genes, could participate to the control of host energy metabolism, and could be relevant for future therapeutic developments.” – “Improvement of obesity and related metabolic disorders by the prebiotic approach. Nutrients with prebiotic properties allows, by changing the gut microbiota, to promote the endocrine function of the gut (increase in GLP-1, and GLP-2 producing cells), and to modulate the activation of the endocannabinoid system in the intestine and in the adipose tissue. All those effects contribute to lessen gut permeability (improved distribution of the tight junction proteins ZO-1 and Occludin), thereby decreasing endotoxemia, and systemic inflammation. Changes in GLP-1 contribute to decrease food intake, fat mass, glycemia and insulin resistance. eCB, endocannabinoid; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2; LPS, lipopolysaccharides; ZO-1, zonula occludens 1.”
The LKM512 strain of yogurt bacteria has attracted particular attention as a probiotic for increasing polyamines levels and inhibiting inflammation.
“Chronic low-grade inflammation is recognized as an important factor contributing to senescence and age-related diseases. In mammals, levels of polyamines (PAs) decrease during the ageing process; PAs are known to decrease systemic inflammation by inhibiting inflammatory cytokine synthesis in macrophages. Reductions in intestinal luminal PAs levels have been associated with intestinal barrier dysfunction. The probiotic strain Bifidobacterium animalis subsp. lactis LKM512 is known to increase intestinal luminal PA concentrations.” (ref)
The 2009 publication Polyamine-rich food decreases age-associated pathology and mortality in aged micerelates polyamine consumption to senescence markers in mice: “The purpose of this study was to test whether oral intake of foods rich in polyamines (spermine and spermidine) suppresses age-associated pathology in aged mice. Synthetic polyamines were mixed into experimental chows, and 24-week-old Jc1:ICR male mice were fed one of three chows containing differing polyamine concentrations. The spermine and spermidine concentrations in the low, normal, and high polyamine chows were 143 and 224 nmol/g, 160 and 434 nmol/g, and 374 and 1540 nmol/g, respectively. An increase in concentration of polyamine in the blood was found only in mice fed the high polyamine chow at 50 weeks of age. While the body weights of mice in all three groups were similar, the survival rate of mice fed high polyamine chow was significantly higher than those in the other two groups (p=0.011). Mice fed the high polyamine chow analyzed at 88 weeks of age, corresponding to the end of the study, demonstrated lower incidence of glomerulosclerosis and increased expression of senescence marker protein-30 in both kidney and liver compared to those fed the low polyamine chow. As these pathological changes are associated with senescence, oral polyamine appears to inhibit the progression of age-associated pathologies.”
The 2009 publication Dynamics of fecal microbiota in hospitalized elderly fed probiotic LKM512 yogurt reports: “The comprehensive dynamics of intestinal microbiota including uncultured bacteria in response to probiotic consumption have not been well studied. The aims of this study were twofold: firstly to analyze the impact on intestinal microbiota of yogurt fermented by Bifidobacterium animalis subsp. lactis LKM512, Lactobacillus delbrueckii subsp. bulgaricus LKM1759, and Streptococcus thermophilus LKM1742 (LKM512 yogurt) and placebo fermented by these lactic acid bacterial strains without LKM512; and secondly to investigate the changes in intestinal microbiota that influence the concentration of PA, one of the beneficial metabolites produced by bacteria in the intestine. The LKM512 yogurt/placebo trial was performed in six hospitalized elderly patients (three men and three women with an average age of 80.3 years) and lasted seven weeks with the following schedule: pre-consumption for one week, LKM512 yogurt consumption for two weeks, washout period for two weeks, and placebo consumption for two weeks. The amount of ingested LKM512 yogurt or placebo was 100 g/day/individual. Fecal samples were analyzed using T-RFLP and real-time PCR. The T-RFLP patterns in five of the six volunteers were changed in a similar fashion by LKM512 yogurt consumption, although these patterns were individually changed following consumption of placebo. It was confirmed that B. animalis subsp. lactis was increased dramatically and Lactobacillus spp. tended to be decreased by LKM512 yogurt consumption. Some indigenous uncultured bacteria were increased and some decreased by LKM512 yogurt/placebo consumption. The similar changes in the intestinal microbiota of the elderly caused by consumption of the LKM512 yogurt were found to be influenced by the LKM512 strain itself, and not by the lactic acid bacteria in the yogurt. Moreover, this study suggests that the increase in intestinal PA concentrations caused by LKM512 yogurt consumption is probably dependent on the LKM512 strain colonizing the intestine.”
Some researchers think targeting the gut microbiota may be an avenue for human life extension.
The December 2011 publication Gut microbiota as a candidate for lifespan extension: an ecological/evolutionary perspective targeted on living organisms as metaorganisms speculates on this possibility: “An emerging central concept in evolutionary biology suggests that symbiosis is a universal characteristic of living organisms that can help in understanding complex traits and phenotypes. During evolution, an integrative circuitry fundamental for survival has been established between commensal gut microbiota and host. On the basis of recent knowledge in worms, flies, and humans, an important role of the gut microbiota in aging and longevity is emerging. The complex bacterial community that populates the gut and that represents an evolutionary adapted ecosystem correlated with nutrition appears to limit the accumulation of pathobionts and infections in all taxa, being able of affecting the efficiency of the host immune system and exerting systemic metabolic effects. There is an urgent need to disentangle the underpinning molecular mechanisms, which could shed light on the basic mechanisms of aging in an ecological perspective. Thus, it appears possible to extend healthy aging and lifespan by targeting the host as a metaorganism by manipulating the complex symbiotic ecosystem of gut microbiota, as well as other possible ecosystems of the body.”
While this discussion is theoretical, it does appear that life extension in mice is possible by feeding them probiotics.
Administration of probiotics can extend the lives of mice.
I was motivated to research and generate this blog entry by coming across the August 2011 publication Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. “Background: Chronic low-grade inflammation is recognized as an important factor contributing to senescence and age-related diseases. In mammals, levels of polyamines (PAs) decrease during the ageing process; PAs are known to decrease systemic inflammation by inhibiting inflammatory cytokine synthesis in macrophages. Reductions in intestinal luminal PAs levels have been associated with intestinal barrier dysfunction. The probiotic strain Bifidobacterium animalis subsp. lactis LKM512 is known to increase intestinal luminal PA concentrations. Methodology/Principal findings: We supplemented the diet of 10-month-old Crj:CD-1 female mice with LKM512 for 11 months, while the controls received no supplementation. Survival rates were compared using Kaplan-Meier survival curves. LKM512-treated mice survived significantly longer than controls (P<0.001); moreover, skin ulcers and tumors were more common in the control mice. We then analyzed inflammatory and intestinal conditions by measuring several markers using HPLC, ELISA, reverse transcription-quantitative PCR, and histological slices. LKM512 mice showed altered 16S rRNA gene expression of several predominant intestinal bacterial groups. The fecal concentrations of PAs, but not of short-chain fatty acids, were significantly higher in LKM512-treated mice (P<0.05). Colonic mucosal function was also better in LKM512 mice, with increased mucus secretion and better maintenance of tight junctions. Changes in gene expression levels were evaluated using the NimbleGen mouse DNA microarray. LKM512 administration also downregulated the expression of ageing-associated and inflammation-associated genes and gene expression levels in 21-month-old LKM512-treated mice resembled those in 10-month-old untreated (younger) mice. Conclusion/Significance: Our study demonstrated increased longevity in mice following probiotic treatment with LKM512, possibly due to the suppression of chronic low-grade inflammation in the colon induced by higher PA levels. This indicates that ingestion of specific probiotics may be an easy approach for improving intestinal health and increasing lifespan. Further studies are required to clarify its effectiveness in humans.” – “When the mice aged or were administered LKM512 treatments, 55 of 93 gene pathways were altered significantly (Z-score >1.98), while the gene expression for 78 of these 93 gene pathways was similar between younger mice and mice receiving LKM512 (Fig. 4C, Fig. S2). Pathways that were downregulated by ageing were upregulated by LKM512 administration and vice versa. In other words, LKM512 administration suppressed ageing-associated change in gene pathways. When the mice aged or were administered LKM512 treatments, 55 of 93 gene pathways were altered significantly (Z-score >1.98), while the gene expression for 78 of these 93 gene pathways was similar between younger mice and mice receiving LKM512 (Fig. 4C, Fig. S2). Pathways that were downregulated by ageing were upregulated by LKM512 administration and vice versa. In other words, LKM512 administration suppressed ageing-associated change in gene pathways.” – “The anti-inflammatory effects of LKM512 administration were also revealed by a DNA microarray. Expression levels of genes in the TNF-NFκB, IL-1, IL-2, and IL-6 pathways were higher in the control group than in LKM512 and younger mice; additionally, gene expression levels in LKM512 mice were similar to those in younger mice (Fig. 5D and Fig. S3).” – “At 25 weeks, 8-OHdG concentrations, which indicate oxidative DNA damage [32], tended to be lower in LKM512 mice than in control mice (P=0.09) (Fig. S4A). Furthermore, pathway analysis using a DNA microarray indicated that the oxidative stress pathway was more active in the control mice than in LKM512 mice (Fig. S4B).”
Final comments
This blog entry provides a general introduction to gut microbiota, their importance to health and to probiotics, prebiotics and synbiotics. And it provides samples from a large and hopeful body of research that might impact significantly on health and longevity. I am impressed that a probiotic diet regimen can extend the lives of mice via a gene activation pattern. However, those of you who follow this blog know that there are a number of other substances that can also do that. In my perception gut microbiota is an important field of research to be followed.
Finally, I want to disclose that for several years I have been taking a symbiotic as part of my personal regimen, one consisting of ten strains of probiotic organisms plus 200 mg of fructooligosaccharides, a prebiotic. And barely a day goes by without my eating a couple of yogurt snacks, ones containing live cultures.

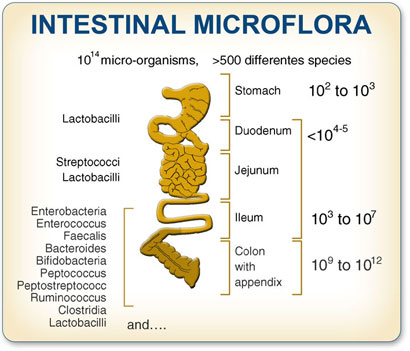
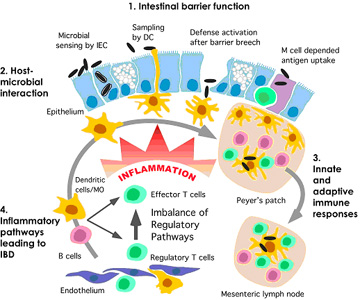
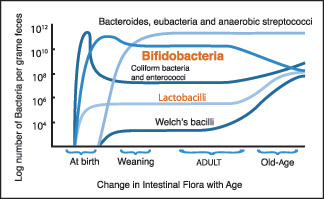

Vince,
Really fascinating stuff on pre, pro and synbiotics.
What probiotic do you take? So many brands out there.
Thank you for a great site!
Hi Dano
I have not researched the best probiotics to buy. I happen most-often to purchase the Vitacost 10-20 product but am open to suggestions.
Vince
Per favore cerchiamo collaborazione ettiva su NUTRA-SCIENZA in Facebook:
Paolo Manzelli
http://www.facebook.com/groups/195771803846822/
Mi piacerebbe molto coloberate con voi. Vi invieremo una e-mail.
Vincenzo
We know there is a complex relationship between diet, gut flora and health. Excessive fructose mainly from sugar and HFCS and grain-based carbohydrates appear to adverse affects on both gut health and overall health. We now believe that these dietary elements also adversely affect brain function, eventually leading to a form of food-induced brain dysfunction we now call Carbohydrate Associated Reversible Brain syndrome or CARB syndrome. People with CARB syndrome can develop up to 22 brain dysfunction symptoms that interfere with your ability to function. The pathology of this disease likely involves changes to the gut flora.
Docww:
An astute comment. Thanks.
Vince
Pingback: Observations on the evolution of evolution | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Quorum sensing Part 1: quorum sensing inhibition via phytochemicals – a new approach against infectious diseases. | AGING SCIENCES – Anti-Aging Firewalls
Pingback: A simple, comprehensive plan to prevent or reverse Alzheimer’s Disease and other neurodegenerative diseases – Part 1: The Plan | AGING SCIENCES – Anti-Aging Firewalls