By Vince Giuliano
This is the second post in a three-part series concerned with new, emerging and potential future treatments for cancers. This Part 2 post is concerned with anti-cancer drug and other interventions that simultaneously address multiple growth pathways. The Part 1 post was concerned principally with interventions that address the mTOR pathway, a growth pathway also of great interest from the viewpoint of longevity. That post also at least partially explains why certain familiar substances like aspirin, coffee, curcumin, resveratrol and green tea may convey both protection against cancers and a longevity benefit.
This and the Part 1 post are about hot areas of intensive research as well as practical clinical experimentation. Many of the papers cited in this blog entry were published in 2012 and a few of them were only a day old when I came across them. Because the experimental treatment regimens described in this and the previous blog entry draw on drugs already approved for cancer treatment or for other indications, clinical usage and experience seems to be increasing rapidly in the areas characterized. The Part 3 blog entry will be concerned with selected less-known phytochemicals that have long been used in traditional Chinese medicine and that in recent years have been subjected to research scrutiny in China using the latest tools of Western Science.
Background
The traditional mainline approaches to treating cancer have been chemotherapy, radiation therapy and surgery, sometimes characterized as: poison, burn and slash. Both chemotherapy and radiation therapy have from the onset been focused on killing cancer cells, in one case by using highly toxic chemicals and in the other case by using focused radiation. Sometimes these approaches have worked and have saved lives. In many instances, however, they have not worked, worked badly, have led to cancer relapses or have contributed to killing patients due to their side effects.
The traditional chemotherapy and radiation approaches had several problems including a) Many chemotherapy agents are extremely toxic to normal cells and using them involves a race to see who dies first, the cancer or the patient, b) It may be difficult to focus the radiation on the tumors concerned and the body may suffer from radiation toxicity, and c) Killing cancer cells may not do significant good if the stem cells for those cancers stay alive and can regenerate the cancer. And d) Another very basic problem is that cancer cells tend to be remarkably clever and naturally seek to take advantage of numerous cellular survival mechanisms to stay alive under stress, for example by upregulating heat shock proteins or DNA repair mechanisms.
These concerns have led to the development of ever-smarter treatments, ones more targeted or that takes advantage of molecular vulnerabilities of cancer cells. See my early (2009) blog posts New-science approaches to detecting, preventing and curing cancers, From four-pound hammer to smart molecules – on cancer treatments, Trojan-horse stem cells might offer an important new cancer therapy, Progress in fighting glioblastoma, On the TRAIL of a selective cancer treatment, and Terminator stem cells in the early pipeline.
There has been increasing concern with understanding the molecular biology and epigenetics of cancers. As the understanding of how key molecular pathways work in cancers has increased, so have more-sophisticated therapies been introduced, often used conjunction with the traditional slash, burn and poison approaches. These include stem cell transplants, use of angiogenesis inhibitors, Dendritic cell cancer immunotherapy, turning P53 on in cancer cells, and adoptive stem cell immunotherapy. This blog entry is concerned with another “smart” approach: simultaneously targeting multiple molecular pathways in cancer cells to defeat their protective responses.
Cancer cell pathways
Starting with basics: “All cancers arise as a result of the acquisition of a series of fixed DNA sequence abnormalities, mutations, many of which ultimately confer a growth advantage upon the cells in which they have occurred. There is a vast amount of information available in the published scientific literature about these changes(ref).” A very large number of gene mutations may be involved in a single cancer – 50 or more – making the molecular dynamics of cancer cells different in important respects from those of normal cells. The COSMICdatabase is a constantly updated catalog of somatic mutations in cancer; it lists 20948 genes and 233349 known mutations.
From Cancerquest: “The abnormal behaviors demonstrated by cancer cells are the result of a series of mutations in key regulatory genes. The cells become progressively more abnormal as more genes become damaged. Often, the genes that are in control of DNA repair become damaged themselves, rendering the cells even more susceptible to ever-increasing levels of genetic mayhem. — Most cancers are thought to arise from a single mutant precursor cell. As that cell divides, the resulting ‘daughter’ cells may acquire different mutations and different behaviors over a period of time. Those cells that gain an advantage in division or resistance to cell death will tend to take over the population. In this way, the tumor cells are able to gain a wide range of capabilities that are not normally seen in the healthy version of the cell type represented.”
Incidence of cancer is heavily correlated with aging
Continuing: “For almost all types of cancer studied to date, it seems as if the transition from a normal, healthy cell to a cancer cell is step-wise progression that requires genetic changes in several different oncogenes and tumor suppressors. This is one reason why cancer is much more prevalent in older individuals. In order to generate a cancer cell, a series of mutations must occur in the same cell. Since the likelihood of any gene becoming mutated is very low, it stands to reason that the chance of several different mutations occurring in the same cell is truly very unlikely. For this reason, the cells in a 70 year old body have had more time to accumulate the changes needed to form cancer cells but those in a child are much less likely to have acquired the requisite genetic changes. Of course, some children do get cancer but it is much more common in older individuals. The graph below shows colon cancer rates in the United States as a function of age. The graph was obtained from the National Cancer Institute(ref).”
A cancer cell may depend on aberrant behavior in multiple molecular pathways. Six of the major pathways that may be involved are diagramed in a high-resolution PDF poster that can be found here. The relevant pathways when detailed are extremely complex and frequently there are multiple routes to get from one point to another.
Colorectal cancer – a case in point
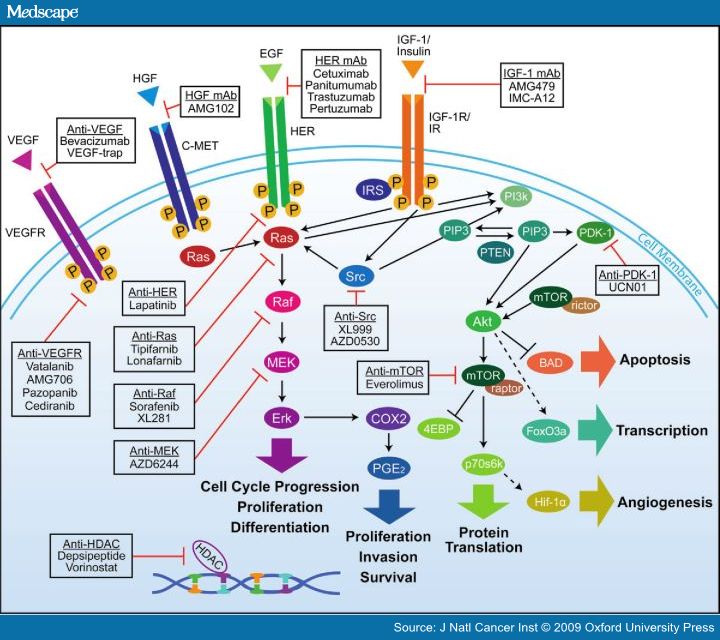
Colorectal cancer is a case in point where a drug can block a single survival pathway but the cancer cells can quickly discover an effective detour and survive. A diagram that relates selected pathways to drug treatments for colorectal cancer and an accompanying explanation of some of the pathways follows.
Source: Medscape. “Figure 1 shows the interactions between various signaling pathways involved in tumor proliferation and progression. Such close interactions between these pathways may provide “escape mechanisms” that allow tumors to circumvent a pathway that has been pharmacologically blocked. — Overview of interlinked cellular signaling pathways involved in the proliferation and progression of colorectal cancer. Agents targeting signaling proteins that have been evaluated or are currently being evaluated in phase II, III, or IV clinical trials for colorectal cancer are shown. The epidermal growth factor receptor (EGFR)–related family of receptor tyrosine kinases includes human epidermal growth factor receptor (HER1), EGFR, or c-erbB1; HER2 or c-erbB2; HER3 or c-erbB3; and HER4 or c-erbB4. C-MET = mesenchymal–epithelial transition factor; EGF = epidermal growth factor; HDAC = histone deacetylases; HGF = hepatocyte growth factor; IGF-1 = insulin-like growth factor-I; IGF-1R = insulin-like growth factor-I receptor; IR = insulin receptor; VEGF = vascular endothelial growth factor; VEGF-R = vascular endothelial growth factor receptor. — The interlinked RAS–MAPK and PI3K signaling pathways — play an important role in tumorigenesis via phosphorylation of various proteins and transcription factors that directly control cell growth, differentiation, and apoptosis.[1,2,30]KRAS, a member of the rat sarcoma virus (ras) gene family of oncogenes (including KRAS, HRAS, and NRAS), encodes the guanosine diphosphate (GDP)– and guanosine triphosphate (GTP)–binding protein RAS that acts as a self-inactivating intracellular signal transducer.[31] After binding and activation by GTP, RAS recruits the oncogene RAF, which phosphorylates MAP2K (mitogen-activated protein kinase kinase)-1 and MAP2K-2, thus initiating MAPK signaling that ultimately leads to expression of proteins playing important roles in cell growth, differentiation, and survival. The oncogene PIK3CA encodes the p110 subunit of PI3K, which can be activated via interaction with RAS proteins.[1,2,30] — Mutation in KRAS, BRAF, or PIK3CA results in continuous activation of the downstream RAS–MAPK or PI3K pathways, regardless of whether the EGFR is activated or pharmacologically blocked. Such activation in turn enhances transcription of various oncogenes, including MYC, CREB, and the gene for nuclear factor κB.[1,2,30] — A recent population-based study of 586 patients with colon adenocarcinomas found mutations in KRAS, BRAF, and/or PIK3CA in 316 (56%) of the 586 tumors studied.[32]KRAS is the most commonly mutated gene in this pathway, with mutations in 35%–45% of colorectal adenocarcinomas; mutations in PIK3CA (≤20%) and BRAF (<15%) are less common.[32–37] Mutations in PIK3CA and KRAS or BRAF may coexist within the same tumor,[32,36–38] but KRAS and BRAF mutations appear to be mutually exclusive[33,34,39–41] (ref).”
Monoclonal antibody cancer therapies that block HER proteins
The HER pathway has to do with activation in cancers of Epidermal Growth Factor (EGF) and is inhibited as shown in the diagram by the drugs Cetuximab, Panitumumab, Trastuzumab and Pertuzumab. These are monoclonal antibodies that bind selectively to HER proteins and compromise the functionality of those proteins. “The HER receptors are proteins that are embedded in the cell membrane and communicate molecular signals from outside the cell to inside the cell, and turn genes on and off. The HER proteins regulate cell growth, survival, adhesion, migration, and differentiation—functions that are amplified or weakened in cancer cells. In some cancers, notably some breast cancers, HER2 is over-expressed, and causes breast cells to reproduce uncontrollably.[1](ref)”
While HER-blocking monoclonal antibody therapies are useful, sometimes they are only weakly effective or won’t work at all as single therapies.
In the case of trastuzumab for example, “However, cancers usually develop resistance to trastuzumab. — The original studies of trastuzumab showed that it improved overall survival in late-stage (metastatic) breast cancer from 20.3 to 25.1 months,[1] but there is controversy over whether trastuzumab is effective in earlier stage cancer.[2] Trastuzumab is also controversial because of its cost, as much as $100,000 per year[3] (ref).” In the case of Cetuximab, “When growth factors bind to their receptors on the surface of the cell, the receptors give a signal that causes cells to divide. Some cancers are caused by mutated receptors that give a signal to divide even without growth factor. That causes the cells to divide uncontrollably. Cetuximab binds to receptors like that and turns off that signal. — The EGFR sends a signal down a pathway that includes another protein, KRAS. In some cancers, the EGFR is mutated. In other cancers, the KRAS protein is mutated, and KRAS sends a signal to divide uncontrollably instead. — Cetuximab binds to EGFR and turns off the uncontrolled growth in cancers with EGFR mutations. However, if the EGFR is normal, and the KRAS protein in mutated, cetuximab won’t work, because the KRAS protein downstream is causing the problem, not the EGFR. — Therefore, before cetuximab is used, the KRAS protein in the cancer cells is tested. If KRAS is normal (wild), cetuximab might work. But if KRAS is mutated, cetuximab won’t work, because KRAS will send a signal to divide even after cetuximab turns the EGFR signal off(ref).”
“Lapatinib—is an orally active drug for breast cancer and other solid tumours.[1] It is a dual tyrosine kinase inhibitor which interrupts the HER2 growth receptor pathway.[2] It is used in combination therapy for HER2-positive breast cancer. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2)(ref).”
A major problem with cancer therapies based in inhibiting the HER pathway is Grb7 upregulation which promotes cancer cell survival and migration. Drug inhibition of Akt signaling is the culprit.
Insight into the cellular feedback loops that limit the effectivenes of HER blocking as a monotherapy is provided by the 2010 publication Grb7 Upregulation Is a Molecular Adaptation to HER2 Signaling Inhibition Due to Removal of Akt-Mediated Gene Repression: “The efficacy of anti-HER2 therapeutics, such as lapatinib and trastuzumab, is limited by primary and acquired resistance. Cellular adaptations that allow breast cancer cell to survive prolonged HER2 inhibition include de-repression of the transcription factor FOXO3A with consequent estrogen receptor activation, and/or increased HER3 signaling. Here, we used low-density arrays, quantitative PCR, and western blotting to determine how HER2 signaling inhibition with lapatinib or PI3K inhibitors affects the expression of genes involved in breast cancer metastatic spread and overall prognosis. Retroviral transgenesis was used to express constitutively active forms of Akt in the HER2(+) breast cancer cell line SKBR3, and Grb7 in MCF7 cells. Specific gene silencing was obtained by siRNAs transfection. A murine BT474 xenograft cancer model was used to assess the effect of lapatinib on gene expression in vivo. We found that lapatinib induces upregulation of Grb7, an adaptor protein involved in receptor tyrosine kinase signaling and promoting cell survival and cell migration. Grb7 upregulation induced by lapatinib was found to occur in cancer cells in vitro and in vivo. We demonstrate that Grb7 upregulation is recreated by PI3K inhibitors while being prevented by constitutively active Akt. Thus, Grb7 is repressed by PI3K signaling and lapatinib-mediated Akt inhibition is responsible for Grb7 de-repression. Finally, we show that Grb7 removal by RNA-interference reduces breast cancer cell viability and increases the activity of lapatinib. In conclusion, Grb7 upregulation is a potentially adverse consequence of HER2 signaling inhibition. Preventing Grb7 accumulation and/or its interaction with receptor tyrosine kinases may increase the benefit of HER2-targeting drugs.” – “ A feedback loop mediated by the PI3K-Akt axis controls Grb7 expression: Grb7 interacts with HER2, participates in HER2 signaling, and promotes cell survival and cell migration. HER2 exerts a repressive control on Grb7 via the PI3K-Akt pathway. Inhibition of HER2 signaling (e.g. by lapatinib) de-represses Grb7 causing its rapid upregulation. Reducing Grb7 with RNAi or preventing its interaction with HER2 using protein-protein interaction inhibitors may help increase the efficacy of anti-HER2 therapeutics and avoid the adverse consequences of Grb7 oncogenic activity.”
Another limitation of EGFR-HER2 inhibition using lapatinib is that insufficient inhibition of PI3K-survivin signaling leads to only a limited pro-apoptotic effect of lapatinib in HER2 amplification-positive cells with a PIK3CA mutation.
The 2011 publication Roles of BIM induction and survivin downregulation in lapatinib-induced apoptosis in breast cancer cells with HER2 amplificationreports: “Lapatinib, a dual tyrosine kinase inhibitor of the epidermal growth factor receptor and human epidermal growth factor receptor 2 (HER2), is clinically active in patients with breast cancer positive for HER2 amplification. The mechanism of this anti-tumor action has remained unclear, however. We have now investigated the effects of lapatinib in HER2 amplification-positive breast cancer cells with or without an activating PIK3CA mutation. Lapatinib induced apoptosis in association with upregulation of the pro-apoptotic protein Bcl-2 interacting mediator of cell death (BIM) through inhibition of the MEK-ERK signaling pathway in breast cancer cells with HER2 amplification. RNA interference (RNAi)-mediated depletion of BIM inhibited lapatinib-induced apoptosis, implicating BIM induction in this process. The pro-apoptotic effect of lapatinib was less pronounced in cells with a PIK3CA mutation than in those without one. Lapatinib failed to inhibit AKT phosphorylation in PIK3CA mutant cells, likely because of hyperactivation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway by the mutation. Depletion of PIK3CA (a catalytic subunit of PI3K) revealed that survivin expression is regulated by the PI3K pathway in these cells, suggesting that insufficient inhibition of PI3K-survivin signaling is responsible for the limited pro-apoptotic effect of lapatinib in HER2 amplification-positive cells with a PIK3CA mutation. Consistent with this notion, depletion of survivin by RNAi or treatment with a PI3K inhibitor markedly increased the level of apoptosis in PIK3CA mutant cells treated with lapatinib. Our results thus suggest that inhibition of both PI3K-survivin and MEK-ERK-BIM pathways is required for effective induction of apoptosis in breast cancer cells with HER2 amplification.”
Regarding HER2 amplication, recalling that HER2 is encoded by the ERBB2 gene, “Amplification or over-expression of the ERBB2 gene occurs in approximately 30% of breast cancers. It is strongly associated with increased disease recurrence and a worse prognosis.[4] Over-expression is also known to occur in ovarian, stomach, and aggressive forms of uterine cancer, such as uterine serous endometrial carcinoma.[5](ref).”
Disappointment with the results of the HER-blocking antibody treatments has thus led to interest in combining these therapies with others especially ones that activate the P13K mTOR pathway. The anticancer effects of blocking mTOR expression were detailed in the Part 1 post of this blog series.
Lapatinib has been used with HER-blocking monoclonal antibodies as a combined cancer treatment.
The 2010 publication Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo reports: “Purpose: HER2 amplification occurs in 18% to 27% of gastric and gastroesophageal junction cancers. Lapatinib, a potent ATP-competitive inhibitor simultaneously inhibits both EGFR and HER2. To explore the role of HER family biology in upper gastrointestinal cancers, we evaluated the effect of lapatinib, erlotinib, and trastuzumab in a panel of molecularly characterized human upper gastrointestinal cancer cell lines and xenografts. Experimental design: EGFR and HER2 protein expression were determined in a panel of 14 human upper gastrointestinal cancer cell lines and HER2 status was assessed by fluorescent in situ hybridization. Dose-response curves were generated to determine sensitivity to lapatinib, erlotinib, and trastuzumab. In HER2-amplified cells, the combination of trastuzumab and lapatinib was evaluated using the median effects principal. The efficacy of lapatinib, trastuzumab, or the combination was examined in HER2-amplified xenograft models. Results: Lapatinib had concentration-dependent antiproliferative activity across the panel with the greatest effects in HER2-amplified cells. There was no association between EGFR protein expression and sensitivity to any of the HER-targeted agents. Cell cycle analysis revealed that lapatinib induced G(1) arrest in sensitive lines and phosphorylated AKT and phosphorylated ERK were decreased in response to lapatinib as well. The combination of lapatinib and trastuzumab was highly synergistic in inhibiting cell growth with a combination index of <1. The combination also induced greater decreases in AKT and ERK activation, G(0)-G(1) cell cycle arrest, and increased rates of apoptosis. In vivo studies showed that the combination of lapatinib and trastuzumab had greater antitumor efficacy than either drug alone. Conclusion: Together, these data suggest that lapatinib has activity in HER2-amplified upper gastrointestinal cancer and supports the ongoing clinical investigation of lapatinib in patients with HER2-amplified disease.”
The combination of trastuzumab and lapatinib may help overcome drug resistance in HER2-positive breast cancers.
The November 2011 publication Different mechanisms for resistance to trastuzumab versus lapatinib in HER2- positive breast cancers — role of estrogen receptor and HER2 reactivation reported: “Introduction: The human epidermal growth factor receptor 2 (HER2)-targeted therapies trastuzumab (T) and lapatinib (L) show high efficacy in patients with HER2-positive breast cancer, but resistance is prevalent. Here we investigate resistance mechanisms to each drug alone, or to their combination using a large panel of HER2-positive cell lines made resistant to these drugs. Methods: Response to L + T treatment was characterized in a panel of 13 HER2-positive cell lines to identify lines that were de novo resistant. Acquired resistant lines were then established by long-term exposure to increasing drug concentrations. Levels and activity of HER2 and estrogen receptor (ER) pathways were determined by qRT-PCR, immunohistochemistry, and immunoblotting assays. Cell growth, proliferation, and apoptosis in parental cells and resistant derivatives were assessed in response to inhibition of HER or ER pathways, either pharmacologically (L, T, L + T, or fulvestrant) or by using siRNAs. Efficacy of combined endocrine and anti-HER2 therapies was studied in vivo using UACC-812 xenografts. Results: ER or its downstream products increased in four out of the five ER+/HER2+ lines, and was evident in one of the two intrinsically resistant lines. In UACC-812 and BT474 parental and resistant derivatives, HER2 inhibition by T reactivated HER network activity to promote resistance. T-resistant lines remained sensitive to HER2 inhibition by either L or HER2 siRNA. With more complete HER2 blockade, resistance to L-containing regimens required the activation of a redundant survival pathway, ER, which was up-regulated and promoted survival via various Bcl2 family members. These L- and L + T-resistant lines were responsive to fulvestrant and to ER siRNA. However, after prolonged treatment with L, but not L + T, BT474 cells switched from depending on ER as a survival pathway, to relying again on the HER network (increased HER2, HER3, and receptor ligands) to overcome L’s effects. The combination of endocrine and L + T HER2-targeted therapies achieved complete tumor regression and prevented development of resistance in UACC-812 xenografts. Conclusions: Combined L + T treatment provides a more complete and stable inhibition of the HER network. With sustained HER2 inhibition, ER functions as a key escape/survival pathway in ER-positive/HER2-positive cells. Complete blockade of the HER network, together with ER inhibition, may provide optimal therapy in selected patients.”
The Part 1 blog entry described how the common drug metformin activates mTOR. The 2009 publication mTOR inhibitors and the anti-diabetic biguanide metformin: new insights into the molecular management of breast cancer resistance to the HER2 tyrosine kinase inhibitor lapatinib (Tykerb)reported: “The small molecule HER2 tyrosine kinase inhibitor (TKI) lapatinib (Tykerb) is approved for the therapy of patients with HER2-positive breast carcinomas who have progressed on trastuzumab (Herceptin). Unfortunately, the efficacy of this HER2 TKI is limited by both primary (inherent) and acquired resistance, the latter typically occurring within 12 months of starting therapy. One of the key factors limiting our understanding of the mechanisms involved in lapatinib resistance is the lack of published preclinical models. We herein review lapatinib-refractory models recently developed at the bench and the survival pathways discovered. As hyperactivation of the pharmacologically targetable PI3K/mTOR/p70S6K1 axis appears to be central to the occurrence of lapatinib resistance, preclinical data showing enhanced antitumour effects when combining lapatinib with mTOR inhibitors (e.g., rapamycin analogues and NVP-BEZ235) highlight the importance of translational work to yield clinically useful regimens capable of delaying or treating lapatinib resistance. The unexpected ability of the anti-type II diabetes drug metformin to inactivate mTOR and decrease p70S6K1 activity further reveals that this biguanide, generally considered non-toxic and remarkably inexpensive, might be considered for new combinatorial lapatinib-based protocols in HER2-overexpressing breast cancer patients.”
The resistance to trastuzumab and lapatinib of breast cancers may be overcome by down-regulating mTOR expression.
The 2008 publication Low-scale phosphoproteome analyses identify the mTOR effector p70 S6 kinase 1 as a specific biomarker of the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb) in human breast carcinoma cells reported on how resistances to HER inhibitors may come about in breast cancer – the culprit being mTOR activation. Further it showed how, on the cell level at least, that combining an mTOR inhibitor with a HER inhibitor increased efficiency of killing cancer cells by a factor of from 10 to 20 compared to the HER inhibitor alone. Background: “Discovery of key proliferative and/or survival cascades closely linked to the biological effects of human epidermal growth factor receptor (HER) 1 (erbB-1) and/or HER2 (erbB-2) inhibitors may identify a priori mechanisms responsible for the development of acquired resistance in breast cancer disease. Here, we took advantage of a semiquantitative protein array technology to identify intracellular oncogenic kinases that distinctively correlate with breast cancer cell sensitivity/resistance to the dual-HER1/HER2 tyrosine kinase inhibitor lapatinib (Tykerb(R)). Materials and methods: MCF-7 cells were forced to overexpress HER2 following stable transduction with pBABE-HER2 retroviruses. The Human Phospho-MAPK Array Proteome Profilertrade mark (R&D Systems) was used to molecularly assess the effects of both the mono-HER2 inhibitor trastuzumab (Herceptintrade mark) and the dual-HER1/HER2 inhibitor lapatinib on 21 different oncogenic kinases. A model of acquired resistance to lapatinib (MCF-7/HER2-Lap10 cells) was established by chronically exposing MCF-7/HER2 cells to increasing concentrations of lapatinib for >10 months. Results: Treatment of MCF-7/HER2 cells with either trastuzumab or lapatinib similarly impaired HER2-enhanced activation status (i.e. phosphorylation) of the mitogen-activated protein kinases, c-Jun N-terminal kinases 1-3 and p38alpha/beta/gamma/delta and of the serine/threonine kinases AKT, glycogen synthase kinase-3, p90 ribosomal s6 kinase1/2, and mitogen- and stress-activated protein kinase1/2. Trastuzumab was less effective than lapatinib at blocking extracellular-signal regulated kinase (ERK) 1/2 and, notably, it failed to deactivate the mammalian target of rapamycin (mTOR) effector p70S6K1. Conversely, lapatinib treatment caused a drastic decrease in the phosphorylation of p70S6K1 at ERK1/2-regulated sites (Thr(421)/Ser(424)) and, as a consequence, p70S6K1 activity measured by its phospho-Thr(389) levels was abolished. The mTOR inhibitor rapamycin was found to supraadditively increase lapatinib efficacy in MCF-7/HER2 cells [ approximately 10-fold enhancement; combination index (CI(50)) = 0.243 < 1.0 = additivity, P < 0.001] but not in p70S6K1 gene-amplified MCF-7 parental cells ( approximately 1.3-fold enhancement; CI(50) = 0.920 congruent with 1.0 = additivity). Lapatinib-resistant MCF-7/HER2-Lap10 cells, which are capable of growing in the continuous presence of 10 microM lapatinib without significant effects on cell viability, notably exhibited a lapatinib-insensitive hyperphosphorylation of p70S6K1. Rapamycin cotreatment suppressed p70S6K1 hyperactivation and synergistically resensitized MCF-7/HER2-Lap10 cells to lapatinib (>20-fold increase in lapatinib-induced cytotoxicity; CI(50) = 0.175 < 1.0 = additivity). Conclusions: Serine-threonine kinase p70S6K1, a marker for mTOR activity that regulates protein translation, constitutes a specific biomarker for the biological effects of the dual-HER1/HER2 inhibitor lapatinib. The clinical implications of our data are that the efficacy of lapatinib might be enhanced with therapies that target the mTOR pathway. Rapamycin analogues such as CCI-779 (Temsirolimus) and RAD001 (Everolimus) may warrant further clinical evaluation to effectively delay or prevent the development of acquired resistance to lapatinib in HER2-positive breast cancer patients.”
However, blocking the mTOR pathway via using a P13K inhibitor may be ineffective as a single therapy for breast cancer because of feedback upregulation of HER3.
“A Phosphoinositide 3-kinase inhibitor (PI3K inhibitor) is a potential medical drug that functions by inhibiting a Phosphoinositide 3-kinase enzyme which is part of the PI3K growth[1][2]/AKT/mTOR pathway, which plays a key role in cancer. Inhibiting this pathway often suppresses tumor (ref).”
Image source Wikipedia
The June 2011 publication Grb7 upregulation is a molecular adaptation to HER2 signaling inhibition due to removal of Akt-mediated gene repressionspeaks again to the need to activate Akt to remove Grb7 for HER inhibitors to be effective against cancers. “The efficacy of anti-HER2 therapeutics, such as lapatinib and trastuzumab, is limited by primary and acquired resistance. Cellular adaptations that allow breast cancer cell to survive prolonged HER2 inhibition include de-repression of the transcription factor FOXO3A with consequent estrogen receptor activation, and/or increased HER3 signaling. Here, we used low-density arrays, quantitative PCR, and western blotting to determine how HER2 signaling inhibition with lapatinib or PI3K inhibitors affects the expression of genes involved in breast cancer metastatic spread and overall prognosis. Retroviral transgenesis was used to express constitutively active forms of Akt in the HER2(+) breast cancer cell line SKBR3, and Grb7 in MCF7 cells. Specific gene silencing was obtained by siRNAs transfection. A murine BT474 xenograft cancer model was used to assess the effect of lapatinib on gene expression in vivo. We found that lapatinib induces upregulation of Grb7, an adaptor protein involved in receptor tyrosine kinase signaling and promoting cell survival and cell migration. Grb7 upregulation induced by lapatinib was found to occur in cancer cells in vitro and in vivo. We demonstrate that Grb7 upregulation is recreated by PI3K inhibitors while being prevented by constitutively active Akt. Thus, Grb7 is repressed by PI3K signaling and lapatinib-mediated Akt inhibition is responsible for Grb7 de-repression. Finally, we show that Grb7 removal by RNA-interference reduces breast cancer cell viability and increases the activity of lapatinib. In conclusion, Grb7 upregulation is a potentially adverse consequence of HER2 signaling inhibition. Preventing Grb7 accumulation and/or its interaction with receptor tyrosine kinases may increase the benefit of HER2-targeting drugs.”
The February 2012 publication Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors reports: “We examined the effects of an inhibitor of PI3K, XL147, against human breast cancer cell lines with constitutive PI3K activation. Treatment with XL147 resulted in dose-dependent inhibition of cell growth and levels of pAKT and pS6, signal transducers in the PI3K/AKT/TOR pathway. In HER2-overexpressing cells, inhibition of PI3K was followed by up-regulation of expression and phosphorylation of multiple receptor tyrosine kinases, including HER3. Knockdown of FoxO1 and FoxO3a transcription factors suppressed the induction of HER3, InsR, IGF1R, and FGFR2 mRNAs upon inhibition of PI3K. In HER2(+) cells, knockdown of HER3 with siRNA or cotreatment with the HER2 inhibitors trastuzumab or lapatinib enhanced XL147-induced cell death and inhibition of pAKT and pS6. Trastuzumab and lapatinib each synergized with XL147 for inhibition of pAKT and growth of established BT474 xenografts. These data suggest that PI3K antagonists will inhibit AKT and relieve suppression of receptor tyrosine kinase expression and their activity. Relief of this feedback limits the sustained inhibition of the PI3K/AKT pathway and attenuates the response to these agents. As a result, PI3K pathway inhibitors may have limited clinical activity overall if used as single agents. In patients with HER2-overexpressing breast PI3K inhibitors should be used in combination with HER2/HER3 antagonists.”
Again, it seems therapeutic intervention with HER, Akt or mTOR signaling via single therapies may well be ineffective. The cancers are just too smart and find work-arounds. So, starting around 2008-2009, it was becoming clear that the thing to try in cancers is simultaneous blocking multiple pathways, for example of HER proteins and Mtor via blocking P13K.
Blocking HER2 and mTOR
The 2010 publiction Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib points out how P13k AKT activation and mTOR inhibition is desirable. “Trastuzumab and lapatinib provide clinical benefit to women with human epidermal growth factor receptor 2 (HER)-positive breast cancer. However, not all patients whose tumors contain the HER2 alteration respond. Consequently, there is an urgent need to identify new predictive factors for these agents. The aim of this study was to investigate the role of receptor tyrosine kinase signaling and phosphoinositide 3-kinase (PI3K)/AKT pathway activation in conferring resistance to trastuzumab and lapatinib. To address this question, we evaluated response to trastuzumab and lapatinib in a panel of 18 HER2-amplified cell lines, using both two- and three-dimensional culture. The SUM-225, HCC-1419, HCC-1954, UACC-893, HCC-1569, UACC-732, JIMT-1, and MDA-453 cell lines were found to be innately resistant to trastuzumab, whereas the MDA-361, MDA-453, HCC-1569, UACC-732, JIMT-1, HCC-202, and UACC-893 cells are innately lapatinib resistant. Lapatinib was active in de novo (SUM-225, HCC-1419, and HCC-1954) and in a BT-474 cell line with acquired resistance to trastuzumab. In these cells, trastuzumab had little effect on AKT phosphorylation, whereas lapatinib retained activity through the dephosphorylation of AKT. Increased phosphorylation of HER2, epidermal growth factor receptor, HER3, and insulin-like growth factor IR correlated with response to lapatinib but not trastuzumab. Loss of PTEN or the presence of activating mutations in PI3K marked resistance to trastuzumab, but lapatinib response was independent of these factors. Thus, increased activation of the PI3K/AKT pathway correlates with resistance to trastuzumab, which can be overcome by lapatinib. In conclusion, pharmacologic targeting of the PI3K/AKT pathway may provide benefit to HER2-positive breast cancer patients who are resistant to trastuzumab therapy.”
The June 2009 publication Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumabreported: “Purpose: Oncogenic activation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway is prevalent in breast cancer and has been associated with resistance to HER2 inhibitors in the clinic. We therefore investigated the combinatorial activity of GDC-0941, a novel class I PI3K inhibitor, with standard-of-care therapies for HER2-amplified breast cancer. Experimental design: Three-dimensional laminin-rich extracellular matrix cultures of human breast cancer cells were utilized to provide a physiologically relevant approach to analyze the efficacy and molecular mechanism of combination therapies ex vivo. Combination studies were done using GDC-0941 with trastuzumab (Herceptin), pertuzumab, lapatinib (Tykerb), and docetaxel, the principal therapeutic agents that are either approved or being evaluated for treatment of early HER2-positive breast cancer. Results: Significant GDC-0941 activity (EC(50) <1 micromol/L) was observed for >70% of breast cancer cell lines that were examined in three-dimensional laminin-rich extracellular matrix culture. Differential responsiveness to GDC-0941 as a single agent was observed for luminal breast cancer cells upon stimulation with the HER3 ligand, heregulin. Combined treatment of GDC-0941, trastuzumab, and pertuzumab resulted in growth inhibition, altered acinar morphology, and suppression of AKT mitogen-activated protein kinase (MAPK) / extracellular signed-regulated kinase (ERK) kinase and MEK effector signaling pathways for HER2-amplified cells in both normal and heregulin-supplemented media. The GDC-0941 and lapatinib combination further showed that inhibition of HER2 activity was essential for maximum combinatorial efficacy. PI3K inhibition also rendered HER2-amplified BT-474M1 cells and tumor xenografts more sensitive to docetaxel. Conclusions: GDC-0941 is efficacious in preclinical models of breast cancer. The addition of GDC-0941 to HER2-directed treatment could augment clinical benefit in breast cancer patients.”
Next, I mention the March 2012 publication Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. “Purpose: The PI3K/Akt/mTOR pathway is an attractive target in HER2 positive breast cancer that is refractory to anti-HER2 therapy. The hypothesis is that suppression of this pathway results in sensitization to anti-HER2 agents. However, this combinatorial strategy has not been comprehensively tested in models of trastuzumab and lapatinib resistance. Experimental design: We analyzed in vitro cell viability and induction of apoptosis in five different cell lines resistant to trastuzumab and lapatinib. Inhibition of HER2/HER3 phosphorylation, PI3K/Akt/mTOR and ERK signaling pathways was evaluated by western blot. Tumor growth inhibition following treatment with lapatinib, INK-128 or the combination of both agents was evaluated in three different animal models: two cell-based xenograft models refractory to both trastuzumab and lapatinib, and a xenograft derived from a patient who relapsed on trastuzumab-based therapy. Results: The addition of lapatinib to INK-128 prevented both HER2 and HER3 phosphorylation induced by INK-128, resulting in inhibition of both PI3K/Akt/mTOR and ERK pathways. This dual blockade produced synergistic induction of cell death in five different HER2 positive cell lines resistant to trastuzumab and lapatinib. In vivo, both cell line-based and patient-derived xenografts showed exquisite sensitivity to the antitumor activity of the combination of lapatinib and INK-128, which resulted in durable tumor shrinkage and exhibited no signs of toxicity in these models. Conclusions: The simultaneous blockade of both PI3K/Akt/mTOR and ERK pathways obtained by combining lapatinib with INK-128 acts synergistically in inducing cell death and tumor regression in breast cancer models refractory to anti-HER2 therapy.”
Note that INK128 is a potent and selective TORC1/2 inhibitor with broad oral antitumor activity.
The February 2012 publication Pharmacologic Inhibition of mTOR Improves Lapatinib Sensitivity in HER2-Overexpressing Breast Cancer Cells with Primary Trastuzumab Resistanceconveys a similar picture, reporting “Lapatinib, a dual EGFR/HER2 kinase inhibitor, is approved for use in patients with trastuzumab-refractory HER2- overexpressing breast cancer. Increased PI3K signaling has been associated with resistance to trastuzumab, although its role in lapatinib resistance remains unclear. The purpose of the current study was to determine if PI3K/mTOR activity affects lapatinib sensitivity. Reduced sensitivity to lapatinib was associated with an inability of lapatinib to inhibit Akt and p70S6K phosphorylation. Transfection of constitutively active Akt reduced lapatinib sensitivity, while kinase-dead Akt increased sensitivity. Knockdown of 4EBP1 also increased lapatinib sensitivity, in contrast to p70S6K knockdown, which did not affect response to lapatinib. Pharmacologic inhibition of mTOR using rapamycin or ridaforolimus increased lapatinib sensitivity and reduced phospho-Akt levels in cells that showed poor response to single-agent lapatinib, including those transfected with hyperactive Akt. Finally, combination mTOR inhibition plus lapatinib resulted in synergistic inhibition of proliferation, reduced anchorage-independent growth, and reduced in vivo tumor growth of HER2- overexpressing breast cancer cells that have primary trastuzumab resistance. Our data suggest that PI3K/mTOR inhibition is critical for achieving optimal response to lapatinib. Collectively, these experiments support evaluation of lapatinib in combination with pharmacologic mTOR inhibition as a potential strategy for inhibiting growth of HER2-overexpressing breast cancers that show resistance to trastuzumab and poor response to lapatinib.”
Histone deacetylase inhibition (HDACi) is another very important emerging approach to cancer therapy, again most-frequently in combination with other pharma approaches
HDAC inhibitors are of interest for treating many medical conditions besides cancers including targeting Alzheimer’s disease(ref), other neurodegenerative conditions(ref), and diabetes(ref). While many substances are HDAC inhibitors including a number of natural ones, the drug panobinostat is being particularly studied and experimentally used in the cancer research community. “Panobinostat (LBH-589) is an experimental drug developed by Novartis for the treatment of various cancers. It is a hydroxamic acid[1] and acts as a non-selective histone deacetylase inhibitor (HDAC inhibitor) — Panobinostat inhibits multiple histone deacetylase enzymes, a mechanism leading to apoptosis of malignant cells via multiple pathways.[1] (ref).[2]”
Another new angle for treating certain cancers is to combine an HER/EGFR inhibitor with a histone deacetylase (HDAC) inhibitor like panobinostat.
The May 2011 publication The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the antitumor activity of the histone deacetylase inhibitor panobinostat in colorectal cancer models reported: “As key molecules that drive progression and chemoresistance in gastrointestinal cancers, epidermal growth factor receptor (EGFR) and HER2 have become efficacious drug targets in this setting. Lapatinib is an EGFR/HER2 kinase inhibitor suppressing signaling through the RAS/RAF/MEK (MAP/ERK kinase)/MAPK (mitogen-activated protein kinase) and PI3K (phosphoinositide 3-kinase)/AKT pathways. Histone deacetylase inhibitors (HDACi) are a novel class of agents that induce cell cycle arrest and apoptosis following the acetylation of histone and nonhistone proteins modulating gene expression and disrupting HSP90 function inducing the degradation of EGFR-pathway client proteins. This study sought to evaluate the therapeutic potential of combining lapatinib with the HDACi panobinostat in colorectal cancer (CRC) cell lines with varying EGFR/HER2 expression and KRAS/BRAF/PIK3CA mutations. Lapatinib and panobinostat exerted concentration-dependent antiproliferative effects in vitro (panobinostat range 7.2-30 nmol/L; lapatinib range 7.6-25.8 μmol/L). Combined lapatinib and panobinostat treatment interacted synergistically to inhibit the proliferation and colony formation in all CRC cell lines tested. Combination treatment resulted in rapid induction of apoptosis that coincided with increased DNA double-strand breaks, caspase-8 activation, and PARP cleavage. This was paralleled by decreased signaling through both the PI3K and MAPK pathways and increased downregulation of transcriptional targets including NF-κB1, IRAK1, and CCND1. Panobinostat treatment induced downregulation of EGFR, HER2, and HER3 mRNA and protein through transcriptional and posttranslational mechanisms. In the LoVo KRAS mutant CRC xenograft model, the combination showed greater antitumor activity than either agent alone, with no apparent increase in toxicity. Our results offer preclinical rationale warranting further clinical investigation combining HDACi with EGFR and HER2-targeted therapies for CRC treatment.”
Combining an mTOR inhibitor and a HDAC inhibitor appears to be an effective way to kill pancreatic cancer cells.
Pancreatic cancer is a deadly cancer for which there is no established treatment. According to a 2009 Mayo Clinic press release Mayo Clinic Researchers Formulate Treatment Combination Lethal To Pancreatic Cancer Cells: “– A combination of two targeted therapies packs a powerful punch to kill pancreatic cancer cells in the laboratory, Mayo Clinic cancer researchers report. — In a study being presented at the AACR 100th Annual Meeting 2009, Mayo Clinic Cancer Center investigators found that rapamycin and panobinostat (also known as LBH589) act synergistically when used in combination, destroying up to 65 percent of cultured pancreatic tumor cells. — The finding is particularly significant, says the study’s first author, Mamta Gupta, Ph.D., because the three cell lines studied were all resistant to the effects of chemotherapy – as are many pancreatic tumors – and because the drugs studied are already available for treatment of patients. Panobinostat is approved as therapy for cutaneous T cell lymphoma (CTCL), and rapamycin is best known as an immunosuppressant to help prevent rejection of transplanted organs. — “We need new therapies and strategies for the treatment of pancreatic cancer because these tumors are resistant to almost all known treatments,” says Dr. Gupta, a research associate in the Division of Hematology. “No targeted treatment has shown much value to date.”
Multiple-pathway cancer treatments are being examined in a large number of clinical trials.
The need for combining cancer therapies addressing multiple pathways is reflected in the large number of clinical trials of such combined therapies. For example, a substantial number of clinical trials are aimed at combinations of Panobinostat with other molecular-based cancer treatments addressing other pathways. There are so many that I list only a sample of them here. The total list can be found using this link:
- A Safety Study of LBH589 (Panobinostat) and RAD001 (Everolimus) to Stabilize Kidney Cancer
- A Dose Finding Study With I.V. Panobinostat (LBH589), Docetaxel, and Prednisone in Patients With Hormone Refractory Prostate Cancer
- Panobinostat Plus Ifosfamide, Carboplatin, and Etoposide (ICE) Compared With ICE For Relapsed Hodgkin Lymphoma
- A Phase II Study of Oral Panobinostat (LBH589) and Rituximab to Treat Diffuse Large B Cell Lymphoma
- Panobinostat (LBH589) Plus Everolimus (RAD001) in Patients With Relapsed and Refractory Lymphoma
- Treatment of Resistant Metastatic Melanoma Using Decitabine, Temozolomide and Panobinostat
- Study of Cisplatin and Pemetrexed in Combination With Panobinostat in Solid Tumors
- Study of Bortezomib and Panobinostat in Treating Patients With Relapsed/Refractory Peripheral T-cell Lymphoma or NK/T-cell Lymphoma
- Panobinostat or Placebo With Bortezomib and Dexamethasone in Patients With Relapsed Multiple Myeloma
- Panobinostat in Combination With Idarubicin and Cytarabine in Patients Aged 65 Years or Older With Newly Diagnosed Acute Myeloblastic Leukaemia (AML)
- Safety and Efficacy Studies of Panobinostat and Bicalutamide in Patients With
Recurrent Prostate Cancer After Castration - A Trial l of Panobinostat Given in Combination With Trastuzumab and Paclitaxel in Adult Female Patients With HER2 Positive Metastatic Breast Cancer
- Panobinostat With Rituximab for Relapsed/Refractory Diffuse Large B Cell Lymphoma
- Carfilzomib Plus Panobinostat in Relapsed/Refractory Multiple Myeloma (MM)
- A Phase II Trial of Panobinostat and Lenalidomide in Patients With Relapsed or
Refractory Hodgkin’s Lymphoma - Study of the Combination of Panobinostat and Carfilzomib in Patients With
Relapsed/Refractory Multiple Myeloma - Phase I Dose Finding and Proof-of-concept Study of Panobinostat With Standard Dose Cytarabine and Daunorubicin for Untreated Acute Myeloid Leukemia or Advanced Myelodysplastic Syndrome
- Panobinostat/Velcade in Multiple Myeloma
Very recent cancer therapy research
Another cancer therapy that may well be coming into practice is monoclonal antibody blocking of the protein CD47.
The January 2012 publication The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors reports: “CD47, a “don’t eat me” signal for phagocytic cells, is expressed on the surface of all human solid tumor cells. Analysis of patient tumor and matched adjacent normal (nontumor) tissue revealed that CD47 is overexpressed on cancer cells. CD47 mRNA expression levels correlated with a decreased probability of survival for multiple types of cancer. CD47 is a ligand for SIRPα, a protein expressed on macrophages and dendritic cells. In vitro, blockade of CD47 signaling using targeted monoclonal antibodies enabled macrophage phagocytosis of tumor cells that were otherwise protected. Administration of anti-CD47 antibodies inhibited tumor growth in orthotopic immunodeficient mouse xenotransplantation models established with patient tumor cells and increased the survival of the mice over time. Anti-CD47 antibody therapy initiated on larger tumors inhibited tumor growth and prevented or treated metastasis, but initiation of the therapy on smaller tumors was potentially curative. The safety and efficacy of targeting CD47 was further tested and validated in immune competent hosts using an orthotopic mouse breast cancer model. These results suggest all human solid tumor cells require CD47 expression to suppress phagocytic innate immune surveillance and elimination. These data, taken together with similar findings with other human neoplasms, show that CD47 is a commonly expressed molecule on all cancers, its function to block phagocytosis is known, and blockade of its function leads to tumor cell phagocytosis and elimination. CD47 is therefore a validated target for cancer therapies.”
Another approach is using Nutlin-3 to de-inhibit P53 expression in cancers.
The April 2012 publication Nutlin-3 induces apoptosis, disrupts viral latency and inhibits expression of angiopoietin-2 in Kaposi sarcoma tumor cellsreports:.”Kaposi sarcoma (KS) tumors often contain a wild-type p53. However, the function of this tumor suppressor in KS tumor cells is inhibited by both MDM2 and latent nuclear antigen (LANA) of Kaposi sarcoma-associated herpes virus (KSHV). Here, we report that MDM2 antagonist Nutlin-3 efficiently reactivates p53 in telomerase-immortalized human umbilical vein endothelial cells (TIVE) that had been malignantly transformed by KSHV as well as in KS tumor cells. Reactivation of p53 results in a G 1 cell cycle arrest, leading to inhibition of proliferation and apoptosis. Nutlin-3 inhibits the growth of “KS-like” tumors resulting from xenografted TIVE-KSHV cells in nude mice. In addition, Nutlin-3 strongly inhibits expression of the pro-angiogenic and pro-inflammatory cytokine angiopoietin-2 (Ang-2). It also disrupts viral latency by inducing expression of KSHV lytic genes. These results suggest that Nutlin-3 might serve as a novel therapy for KS.”
Minocycline might be an anti-cancer therapy.
Minocycline, a traditional antibiotic, has recently been found to be effective against one type of cancer. The January 2012 publication Minocycline inhibits growth of epithelial ovarian cancer reports “.Objective: These studies were designed to determine whether minocycline inhibits ovarian cancer growth in vitro and in vivo and the molecular mechanisms involved. Materials And Methods: The effect of minocycline on ovarian cancer cell proliferation, cell cycle progression and apoptosis was assessed using human ovarian cancer cell lines OVCAR-3, SKOV-3 and A2780. Then, the capacity of minocycline to inhibit growth of OVCAR-3 xenografts in female nude mice was examined. Results: Minocycline inhibited cell proliferation and colony formation, down-regulated cyclins A, B and E leading to arrest of cells in the G(0) phase of the cycle and suppression of DNA synthesis. Furthermore, exposure of these cells to minocycline led to DNA laddering, activation of caspase-3 and cleavage of PARP-1. In nude mice bearing sub-cutaneous tumors, minocycline suppressed tumor proliferation index, angiogenesis and tumor growth. Conclusion: These findings provide the initial basis for further evaluation of minocycline in the treatment of ovarian cancer.”
If and as any if these new therapies come into clinical use it seems a good bet that it will be used in combination with existing therapies: blocking mTOR, blocking HER pathways, applying histone deacetylase inhibitors, etc..
Wrapping it up
As of now, the list of existing and emerging cancer therapies discussed in this blog are:
- P53 upregulation in cancers (ref),( this post)
- Stem cell virotherapy (ref)
- Use of TRAIL (ref)(ref)
- mTOR inhibition (ref)
- Dendritic cell cancer immunotherapy (ref)
- SIRT3 AND PGC-1alpha (ref)
- CAR adoptive stem cell immunotherapy (ref)
- HER channel suppression (this post)
- Blocking CD47 (this post)
- Minocycline therapy (this post)
- Use of histone deacytelase inhibitors (this post)
- Use of phytochemicals for prevention or therapy (ref)(ref)(ref)(ref)(ref)(ref)(ref)(ref)(ref)(ref)
- Familiar dietary substances (ref)
There is much more to current research on cancers than I have been able to report on here. The main points of this blog entry are 1. that as molecular pathways in cancers are becoming more understood, molecular therapies that address vulnerabilities in cancer cells are being identified and developed, 2. cancer cells are smart and tend to develop work-arounds that provide resistance against individual targeted drugs. 3. An important tendency in cancer therapies is to hit cancers simultaneously with multiple targeted drugs that address multiple biological pathways.
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION.



Pingback: New, emerging and potential treatments for cancers: Part 1 – focus on the mTOR pathway | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Plant polyphenols –six epigenetic knockout punches against cancers | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Plant polyphenols – six epigenetic knockout punches against cancers | AGING SCIENCES – Anti-Aging Firewalls