By James Watson and Vince Giuliano
This is the first part of a three-part series of blog entries on the epigenetic’s of cancer and aging and how those two deadly dragons can be seriously slow down or stopped with the assistance of plant polyphenols. This Part 1 blog entry will 1. Identify similarities in the biological processes and epigenetic’s of cancer and aging, 2. Identify therefore how common strategies might be found that address both cancer and aging. 3. Describe the process of Xenohormesis whereby stress response chemicals developed over millions of years in plants keep plants healthy can do the same in humans. 4. Provide molecular explanations for the “causality” of cancer and aging, 5. Describe the processes in cancer and aging of epigenetic silencing of “good” genes and epigenetic activation of “bad” genes, 6. Identify a 3 tiered “Pyramid” approach for chemoprevention of aging and cancer, 7. Identify the exact interventions involved in each layer of the Pyramid, and 8. Identify how the interventions in the three layers of the Pyramid can be integrated together.
The Part 1 blog entry contains the main messages and the materials in the Part 2 and Part 3 blog entries in this Two-Dragon series provide additional detail, essentially defining a series of appendices to the Part 1entry, They’re published separately because of blog length considerations and because they are of interest in their own right. The Part 2 blog entry is concerned in more detail with the silencing of good genes in aging and cancer and how many plant polyphenols can prevent that. The Part 3 blog entry is concerned with a. the “unsilencing of bad genes” with sirtuin decline and the harmful results in aging and cancer, and B. providing a master list of drugs and natural compounds for cancer chemoprevention.
The High Lifetime Risk of Cancer – occurrence: 43% in males, 38% in females death risk: 1:4 for males, 1:5 for females
According to the most recent data from US National Cancer Institute’s SEER database, the lifetime risk of developing cancer is 44.81% in males and 38.17% in females. This is higher than for any other disease except for the age-related diseases (described below). The risk of dying from cancer is 1 in 4 for males and 1 in 5 for females. This results in a large number of “lost years” due to premature death. For this reason, developing a cancer risk reduction program should be seriously pursued in a similar fashion to the successful cardiovascular risk reduction programs that have resulted in a dramatic increase in survival over the past 30 years (diet, exercise, statins, anti-platelet therapy, ACE inhibitors, β-blockers, fish oil, and ATreceptor blockers). Unfortunately, very little chemoprevention has entered clinical practice.
The “Missing Causality” of Cancer – cancer etiology: Genetic causes + Environmental causes + Lifestyle causes ≠ 100%
Many causes for cancer have been discovered, based on the study of genetics, epidemiology, toxicology, mutagenesis, and radiation biology. This has led to the discovery of many risk factors for cancer including inherited mutations, single nucleotide polymorphisms, environmental pollutants, ionizing radiation, UV light, etc. Despite an extensive study of the genetic and environmental causes of cancer, the evidence suggests that lifestyle factors play a greater role in the etiology of cancer than all genetic and environmental causes combined. These lifestyle factors include smoking, obesity, sedentary lifestyle, high fat diet, low consumption of fruits and vegetables, alcohol consumption, red meat consumption, and hormone replacement therapy. When these genetic, environmental, and lifestyle risk factors are mathematically quantified, however, they do not add up to 100%. For most cancers, these 3 categories account for less than 50% of the total cases. For instance, with breast cancer, inherited gene mutations account for less than 5% of the total cases; environmental risk factors account for less than 10% of cases; and lifestyle factors account for an estimated 30% of the total cases, which only add up to 40-45%. Adding the contribution of single nucleotide polymorphisms (SNPs) that increase “gene-determined cancer susceptibility”, we can only account for 50% of the “cause” of breast cancer. What is missing ? Most believe it is epigenetics.
The High Lifetime Risk of Aging & Age-related diseases – aging etiology: Genetic causes + Environmental causes + Lifestyle causes ≠ 100%
The only category of diseases with a higher lifetime risk than cancer is aging and age-related diseases. Age-related diseases include both those recognized as “formal diseases” by the FDA (cataracts, AMD, osteoporosis, Alzheimer’s disease, osteoarthritis, hearing loss, etc.) and those that are still unrecognized as “formal diseases” by the FDA (skin aging, sarcopenia, osteopenia, fat atrophy, and aging in general). Obviously, the incidence of aging is 100% and the incidence of these age-related diseases approaches 100% as one ages, but the time of onset varies greatly due to various factors. Part of the etiology of aging and age-related diseases is known and includes genetic mutations that predispose a person to premature aging (Hutchinson-Gilford progeria, Progeroid syndromes, Werner’s syndrome, Ataxia-Telangiectasia, etc.), environmental factors (UV light exposure, XRT, toxins), and lifestyle factors (obesity, smoking, lack of exercise, alcohol consumption, hormone use, high fat diet, etc.). Much like cancer, however, these known causes of aging do not add up to 100%. This again begs the question – “What is missing”. Most believe that it is epigenetics.
The “Missing Causality” of Aging
Many explanations for aging have been proposed, based on the study of model organisms, accelerated aging diseases, knock-out models, exceptionally long-lived organisms, and transgenic model organisms. Genetic and epigenetic studies have also revealed many more clues. Despite all this data, well over a dozen major theories of aging persist, all of which do not completely explain the findings. You can, for example, see the descriptions of 17 such theories in Vince’s treatise ANTI-AGING FIREWALLS – THE SCIENCE AND TECHNOLOGY OF LONGEVITY.
In an attempt to sort out the causes of aging, the terms “intrinsic aging” and “extrinsic aging” have been coined. Extrinsic aging includes factors that are due to external, environmental causes such as UV light, radiation exposure, obesity, smoking, dietary factors, and toxins. Intrinsic aging describes events that occur within a cell that are not due to external factors. Intrinsic aging is the area where most researchers believe the answers to the “missing causality” of both cancer and aging will be found. One of the most effective tools to study intrinsic aging has been the study of Caloric restriction (CR)(ref)(ref). CR has shown a longevity effect and a health benefit in all organisms except for primates, where CR improves healthspan but may not increase lifespan. CR appears to be a series of adaptive responses to nutritional deprivation where 5 major stress-coping pathways are involved [ (-)mTOR, (-)Insulin/IGF-1, (+) AMPK, (+)SIRT, (+)Autophagy, (+)Mitochondrial biogenesis, (+) ribosome biosynthesis, (-) inflammation]. Other cellular stress adaptation pathways not involved with CR have been identified that confer a longevity advantage as well (electrophile response, xenobiotic response, hypoxia response, unfolded protein response, heat shock response, cold shock response, and the DNA damage response). A robust adaptive response to all of these stressors is associated with longevity and health. Several other blog entries have discussed this adaptive response phenomenon known as hormesis(ref)(ref). Unfortunately, the response to all these stressors declines with aging. Evidence is slowly accumulating, suggesting that epigenetic silencing of important gerosuppresor genes and stress coping genes is the cause.
Cellular Senescence – a cellular explanation for aging,& its paradoxic effects on cancer: Good: cancer tumor suppressor mechanism Bad: a cancer promoting mechanism via SASP
The discovery that chromosomes have non-coding DNA “caps” called telomeres at their ends and that telomeres shorten with cell division led to the theory that telomeres were like an “aging clock” with a finite number of ticks on the clock. The study of telomere shortening led to the discovery of another phenomena called cellular senescence (CS). Senescent cells displayed all the classic signs of aging and telomere shortening was once hailed as the central cause. Unfortunately, the telomere shortening theory fell apart when other causes of CS were discovered that were independent of telomere length. (See the discussion in the aforementioned treatise under the subheading An evolving perspective on the Telomere shortening theory of aging and also the discussion in this blog entry.) CS (not telomere shortening) is now considered to be a central cause of aging and a tumor suppressor mechanism used to turn the cell cycle off in cancer cells(ref). CS also explains how chemotherapy and radiation therapy can successfully treat cancer by inducing CS, without killing all of the cancer cells. The beneficial effects of CS in halting the development of cancer and its role in wound healing are the two prime reasons why we do not want to abolish CS pathways. On the other hand, getting rid of senescent cells would be a good thing for preventing aging and preventing the recurrence of inflammation-driven cancers, since senescent cells are the source of these inflammatory cytokines (the SASP) that drive the epithelial-to-mesenchymal transition, which is a key step in carcinogenesis(ref). This is the paradoxical effect of CS.
The “Two Dragons” are More alike than they are Different – cancer and aging share more similarities than differences
Although there are major differences between aging and cancer, they both end up on the same “dead end” street! From a proliferative point of view, they are clearly opposites.
Cancer phenotypes all manifested an uncontrolled cell cycle and uncontrolled growth. Aging phenotypes are manifested as cellular senescence, which is “cell cycle arrest”. In most other aspects, however, cancer cells and senescent cells are actually very similar. Here is a list of some of these similarities:
| Cellular Phenomena | Cancer Cells | Senescent Cells |
| Inflammation | upregulated | upregulated |
| Apoptosis | apoptosis resistant | apoptosis resistant |
| Telomeres | short | short |
| Inflammation | high | high |
| Mitochondria | damaged | damaged |
| Reactive oxygen species | high levels | high levels |
| Antioxidant enzymes upregulated | yes | yes |
| Inflammatory cytokine secretion | yes – IL-6 driven | yes – IL-6 driven (SASP) |
| Immune evasion | yes –TGF-β driven | yes – TGF-β driven |
| Defective DNA repair | yes | yes |
| DNA mutations | present | present |
| Harmful epigenetic silencing | yes | yes |
| Harmful epigenetic desilencing | yes | yes |
| mTOR upregulated | yes | yes |
| Ras/Raf pathway upregulated | yes | yes |
| c-myc pathway upregulated | yes | yes |
| Angiogenesis upregulated | yes | yes |
| Extracellular matrix degradation | upregulated | Upregulated |
You can also check out the discussion in the 2011 publication Cellular senescence: A link between cancer and age-related degenerative disease?
Finding Common Solutions to the Problem of Aging and Cancer – avoiding strategies with antagonistic pleiotropy effects
|
How To Kill Two Dragons with One Stone Interventions that both prevent cancer and aging would be the most effective therapy for increasing average lifespan. Proposed therapies that would fit into this portion of the overlapping circles would include mTOR inhibition, inhibition of the Insulin/IGF-1 pathway, and inhibition of inflammatory pathways (NF-kB, COX, LOX, etc.). Activating the following pathways would also reduce cancer and increase longevity: AMPK , FOXO, Nrf2, PGC-1a, autophagy, ribosomal biosynthesis, nuclear laminin maintenance, DNA repair pathways, and epigenetic mechanisms that maintain normal gene expression (DNMT inhibition, HDAC inhibition, HAT inhibition, miRNA maintenance, chromatin maintenance in the euchromatin state, and preventing epigenetic drift). Clearing senescent cells and maintaining healthy/normal numbers of stem cells would also solve both. |
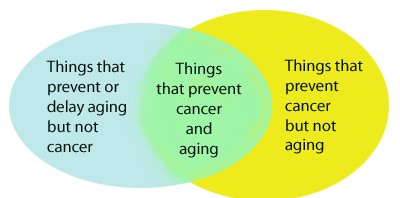
If cancer and aging have more similarities than differences, there is reason to believe that many strategies that prevent aging could also prevent cancer. Since 20-25% of humans will die of cancer, overall average life span would also be dramatically increased with the reduction of cancer occurrence, even if the maximum life span did not increase with these strategies. There are many other therapies that may decrease cancer, but that also increase aging. Examples of this would be the typical treatment for cancer – chemotherapy and radiation. Both of these therapies have been shown to accelerate aging. Likewise, there are therapies that may be considered “anti-aging”, but increase cancer risk. This includes the use of exogenous hormone supplementation. Although certain hormones like HGH appear to reverse some of the undesirable effects of aging, such as muscle atrophy, such hormone supplementation in old age can actually accelerate aging and increase the risk of cancer. Telomerase activators may have the same effects. They may decrease cellular senescence but increase cancer in rodents. For this reason, we propose that anti-cancer therapies and anti-aging therapies can be best understood by the following Venn diagram:
The Concept of Xenohormesis
Xenohormesis is the concept that different species such as plants and animals have common stress signaling molecules, and that such molecules can be harvested from plants and used to increase stress adaptation pathways in animals(ref)(ref)(ref). Plants cannot run away from predators, parasites, infectious agents, hot weather, or cold weather. For this reason, plants have evolved a large number of molecular stress coping pathways that are activated by compounds that are actively synthesized in response to the stressor. Some of these compounds ward off predators with bitter tasting compounds. Others ward off potential organisms that would eat the plant by synthesizing poisons that would kill the predator (i.e. natural pesticides). Although there are many toxic compounds in this arsenal of phytochemicals, there is a large family of molecules called polyphenols that are non-toxic and appear to have great benefits in humans. Approximately 14,000 of these plant-based stress-signaler polyphenol compounds have been discovered so far in plants. They are found in the leaves, stems, flowers, seeds, fruits, nuts, and shells surrounding the nuts. These plant polyphenols appear to be xenohormetic compounds in that they also upregulate stress coping pathways in mammalian cells. These xenohormetic compounds appear to prevent aging and cancer through a large number of pathways. For this reason, their mechanism of action is multifactorial or pleiotropic. Xenohormetic compounds include resveratrol, curcumin, EGCG, isothiocyanates, secoiridoids, genistein, gallic acid, lycopene, allyl mercaptan, plumbagin, etc. Multiple plant polyphenols and their mechanisms of action have been reviewed in past entries in this blog. See for example ref, ref, ref, ref, ref, ref, ref and ref.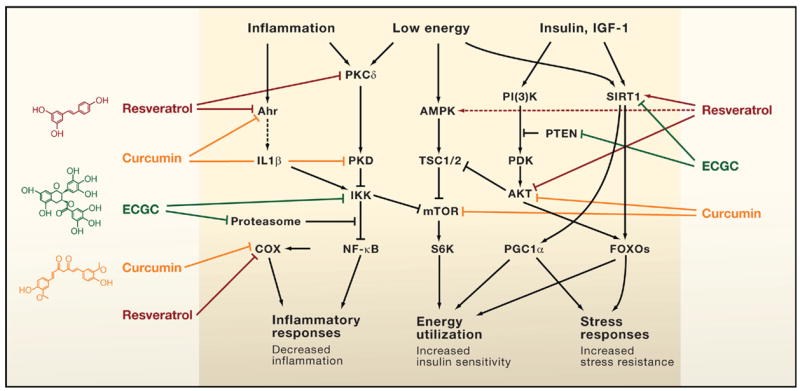
Direct Modulation of Key Mammalian Enzymes by Plant Metabolites
A surprising number of plant molecules in our diet interact with key regulators of mammalian physiology to provide health benefits. Shown are three examples: resveratrol found in numerous plants and concentrated in red wine; curcumin from turmeric; and epigallocatechin-3-gallate (EGCG) in green tea. These compounds modulate key pathways that control inflammation, the energy status of cells, and cellular stress responses in a way that is predicted to increase health and survival of the organism. Such observations raise the question, are these biochemical interactions merely a remnant of what existed in the common ancestor of plants and animals, or is selection maintaining interactions between the molecules of plants and animals? Some interactions activate signaling pathways (arrows) whereas others inhibit them (bars). Solid arrows or bars indicate instances where there is some evidence of a direct interaction of the plant metabolite with a mammalian protein(ref)”A mechanism of xenohormesis appears to be animal gene regulation mediated by plant micro RNAs acquired through food intake.
The 2011 publication Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA reports: “Here, we report the surprising finding that exogenous plant miRNAs are present in the sera and tissues of various animals and that these exogenous plant miRNAs are primarily acquired orally, through food intake. MIR168a is abundant in rice and is one of the most highly enriched exogenous plant miRNAs in the sera of Chinese subjects. Functional studies in vitro and in vivo demonstrated that MIR168a could bind to the human/mouse low-density lipoprotein receptor adapter protein 1 (LDLRAP1) mRNA, inhibit LDLRAP1 expression in liver, and consequently decrease LDL removal from mouse plasma. These findings demonstrate that exogenous plant miRNAs in food can regulate the expression of target genes in mammals.” The miRNAs are present in microvesicles in the plasma where they are circulated to various cell types and serve as intercellular signaling molecules. “Our further studies demonstrated that miRNAs could be selectively packaged into MVs and actively delivered into recipient cells where the exogenous miRNAs can regulate target gene expression and recipient cell function15. Thus, secreted miRNAs can serve as a novel class of signaling molecules in mediating intercellular communication15. The novel and important functions of the secreted miRNAs were also reported by many other groups18,19,20,21. The identification of circulating miRNAs, mainly delivered by cell-secreted MVs, as stable and active signaling molecules opens a new field of research in intercellular and interorganelle signal transduction.”
From a 2011 article in The Scientist about this research Plant RNAs Found in Mammals: “To test his hypothesis, Zhang and his team of researchers sequenced the blood microRNAs of 31 healthy Chinese subjects and searched for the presence of plant microRNAs. Because plant microRNAs are structurally different from those of mammals, they react differently to oxidizing agents, and the researchers were able to differentiate the two by treating them with sodium periodate, which oxidizes mammal but not plant microRNAs. — To their surprise, they found about 40 types of plant microRNAs circulating in the subjects’ blood—some of which were found in concentrations that were comparable to major endogenous human microRNAs. — The plant microRNAs with the highest concentrations were MIR156a and MIR168a, both of which are known to be enriched in rice and cruciferous vegetables such as cauliflower, cabbage, and broccoli. Furthermore, the researchers detected the two microRNAs in the blood, lungs, small intestine, and livers of mice, in variable concentrations that significantly increased after the mice were fed raw rice (although cooked rice was also shown to contain intact MIR168a). — Next, the researchers scoured sequence databases for putative target genes of MIR156a and MIR168a and found that MIR168a shared sequence complementarity with approximately 50 mammalian genes. The most highly conserved of these sequences across the animal kingdom was the exon 4 of the low-density lipoprotein receptor adapter protein 1 gene (LDLRAP1).”So, stress-responsive phytochemicals consumed by humans activate similar evolutionary-conserved stress pathways in mammals, including us. “The exogenous mature plant miRNAs in food can pass through mouse GI tract and enter the sera and organs” and “Plant miRNAs execute their function in mammalian cells in a fashion of mammalian miRNA(ref).” The plant’s hormetic-acting chemical stress protection mechanisms developed over millions of years become part of ours.
Molecular Explanations for the “Causality” of Cancer and Aging
Since genetic studies of inherited cancer, genome-wide association studies, epidemiology studies, toxicology studies, and other approaches to studying “causality” have failed to mathematically account for the entire incidence of cancer, a “ground up” investigation of carcinogensis has yielded a plethora of explanations for cancer “causality”. Most of this has been done with in vitro studies of cancer cells, where they are exposed to various synthetic and natural compounds that activate/inhibit a specific molecular pathway. This has resulted in the discovery of the following molecular explanations for cancer. These pathways overlap, intersect, or merge at various points. For this reason, this list should not be viewed as separate mechanisms of tumorigenesis, but rather, different ways of explaining the same phenomena – cancer. Cancer embodies all of these cellular defects which result in uncontrolled cell division, apoptosis resistance, dependence1.
Epigenetic Silencing of “Good Genes”
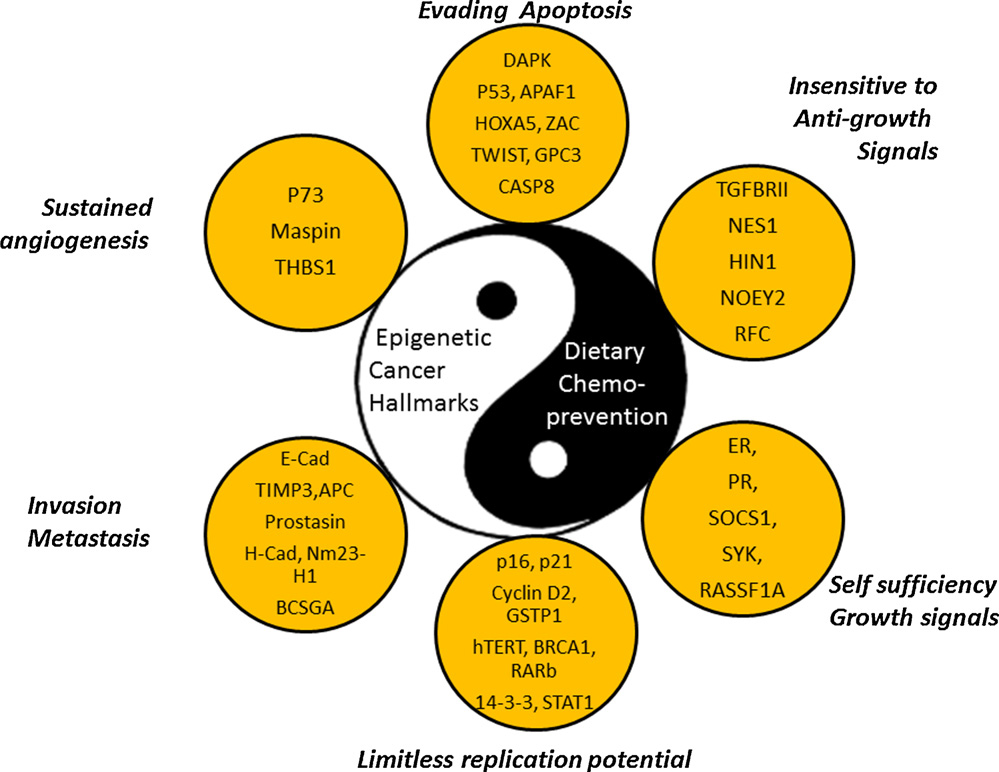
We now know that in many cases, a DNA mutation does not have to occur for cancer to develop. Instead, the non-mutated gene can be silenced by an epigenetic mechanism that involves histone protein deacetylation of active genes (euchromatin), as well as DNA methylation of cytosine residues at “start sites” on the gene (the “start site” is called the promoter site). The combination of histone deacetylation and promoter site DNA methylation results in the inability of a transcription factor to access the gene and to bind to the promoter site. With cancer, over 600 genes have been found to be silenced by this epigenetic mechanism. Here is an illustration showing the 6 groups of genes that if silenced, the cell can form cancer without any DNA mutations. The mechanisms of silencing are the same as that seen in aging.
The Six Hallmarks of Cancer (And why DNA sequencing is not enough to Diagnose the Defects in Cancer)
Cancer is often thought of as just being a cell that will not stop dividing, a cell that has lost control over the cell cycle. This is true, but there are actually 6 key features of cancer, and cell cycle control is only one of these. The diagram lists these 6 characteristics of cancer cells and the abbreviations of specific genes that if silenced, allow the cell to turn into cancer. All of these genes have been identified in various cancers to be silenced or mutated, however they do not all have to be silenced or mutated for the cell to turn into cancer. Only 1 or 2 of these genes in each category must be silenced or mutated. Silencing usually occurs due to promoter site (CpG island) methylation, but only 50% of genes have CpG islands. All genes can be epigenetically silenced by histone deacetylation, however. Another form of gene silencing is called polycomb protein silencing. Another form of silencing involves non-coding RNA called miRNA which prevent the translation of messenger RNA or increase the degradation of mRNA. DNA mutations also effectively turn off these genes, but mutations do not account for the number of cellular changes seen with cancer. This is why gene sequencing of cancer cells does not always reveal what genes are not working. The only way to truly figure this out is to do gene sequencing, epigenetic sequencing, and RNA profiling of cancer.
Examples of Epigenetic Silencing
1a. DNA repair gene inactivation by epigenetic silencing – Four examples of this are the genes hMLH1, MGMT, WRN, and BRCA1h.
MLH1 – this an important gene for DNA mismatch repair, which is the way that microsatellite-unstable regions are repaired to avoid them becoming bigger. Gene inactivation by CpG island hypermethylation has been observed at the promoter site for this gene
MGMT – this is an important gene for fixing mutant guanine bases that become chemically modified by a methyl or alkyl group. This modification results in the “G” being read as a “A” (i.e. creating a G-to-A single nucleotide polymorphism). MGMT removes the promutagenic guanine residue. Unfortunately, this gene is inactivated by CpG island hypermetylation with aging.
WRN – this is the gene responsible for Werner’s syndrome. This gene codes for the WRN protein which has helicase and exonuclease activity. With aging, the CpG island at the promoter site for this gene is hypermethylated, effectively silencing this gene. As a consequence, the manifestations are a progeroid phenotype and increased risk of cancer due to extreme sensitivity to DNA damaging drugs or toxins.
BRCA1 – this gene is the most common gene that is mutated with hereditary breast cancer. However in sporadic cases of breast and ovarian cancer, the BRCA1gene can be silenced by CpG island hypermethylation in the promoter site of this gene.
b. Progeroid syndromes and atypical Werner’s syndrome – inactivation by epigenetic silencing. Two examples of accelerated aging genes – Lamin A/C and WRN.
Laminin A/C – Mutations in the Laminin A/C gene produce a syndrome called Hutchinson’s- Gilford progeria. Although most progeroid syndromes are due to DNA mutations, we now know that a progeroid-like phenomena can be acquired due to epigenetic silencing of the same gene that is mutated in the hereditary form of HG progeria. The Laminin A/C gene codes for two different laminin proteins that make up the scaffolding just inside the nuclear double membrane. When this gene is hypermethylated at the promoter site, a syndrome called “atypical Werner’s syndrome” occurs.
WRN – again, this is called the “Werner gene” and is mutated in classic Werner’s syndrome (WS). Patients with WS develop accelerated aging and manifest cataracts, type II diabetes, osteoporosis, arteriosclerosis, and cancer at an earlier age and at increased incidence. There are cases of WS where the only problem is CpG island hypermethylation at the promoter site site. Again, this is indicative of a need for a “DNA methylome” for proper diagnosis.
1c. Alzheimer’s disease – Gene inactivation by epigenetic silencing
2. Global hypomethylation of the genome – This is also a feature of cancer and aging that is responsible for activating endoparasitic DNA (i.e. retrotransposons). This results in global genomic instability, which leads to DNA mutations, cancer, and age-related diseases.
3. Decline in NAD+ Redox Sensors (Sirtuin Deacetylases
a. Nuclear Effects of Sirtuin Decline: Epigenetic expression of genes that should be silenced (rDNA locus) – 20 to of SIRT2 & SIRT6 histone deacetylation
Normal Gene silencing
SIRT2 – global deacetylation of lysine 16 on H4 gene silencing – tenovin-6 inhibits this silencing genes expressed that should be silenced
SIRT6 – global deacetylation of lysine 9 and 14 on H3 genes silencing – tenovin 6 inhibits this silencing genes expressed that should be silenced
Consequence of age-induced loss of gene silencing:
rDNA repeat recombination accumulation of extrachromosomal rDNA circles, aging or cell death
telomeres are not silenced
b. Cytoplasmic Effects of Sirtuin Decline: gene expression for stress resistance and cancer prevention – 20 to SIRT1 transcription factor deacetylation
Normal cancer prevention and aging prevention
SIRT1 – cancer prevention via 5 ways: (+) cancer-specific apoptosis by deacetylating survivin, (-) cancer growth by deacetylating β-catenin, (-) cancer angiogenesis by deacetylating Notch, (+) genomic stability via deacetylating histones and NBS1,
SIRT1 – (-) ROS damage to DNA via deacetylation leading to Nrf2 and FOXO3, ARE expression
SIRT1 – stress resistance by deacetylating p53, FOXO, Ku70 transcription factors, less apoptosis of healthy cells, stress resistance (this is lost with aging)
Consequence of age-induced loss of SIRT1 activity: acetylated p53 induces apoptosis (nonacetylated p53 inhibits apoptosis)
c. Mitochondrial Effects of Sirtuin Decline: mitochondrial biogenesis, fatty acid oxidation, mito respiration, acetate usage, and amino acid usage
Normal mitochondrial effects increasing metabolism & energy production:
SIRT1: PGC-1α deacetylation, mitochondrial biogenesis, fatty acid oxidation and respiration
SIRT3 Complex I synthesis; respiration; Ace-CS2 acetate usage; GDH amino acid usage
SIRT4 GDH amino acid usage
SIRT5 CPS1 amino acid usage
4. Decline in AMP Energy Sensor Activity (AMP Kinase)
a. Cytoplasmic Effects of AMPK Decline: less active AMPK
5. Increase in mTOR kinase Activity
6. Decline in Mitochondrial Biogenesis
7. Decline in Autophagy (-) autophagy or failure of autophagy to work adequately
– mTOR overactivation,
– AMPK inhibition
– inability of SIRT1 to induce autophagy with starvation
8. Antagonistic Pleiotropy Pathways – Over- activation pathways that promote growth when you are young and aging when you are old:
a. Insulin/IGF-1/PI3K/Akt/FOXO
b. mTOR/S6k
c. Protein kinase A
d. Protein kinase B
e. Protein kinase C
f. c-Myc
g. Ras/Raf/MEK/ERK
h. Ras/PI3K/PTEN/Akt/mTOR
9. Inflammatory pathway up regulation
a. Inflammation due to PPAR inhibition – Chronic inhibition of PPARα,γ elements (PPREs) => stimulation of inflammation via NF-kB, AP-1, STAT, an NFAT pathways
b. Inflammation due to IL-6 – Chronic IL-6 induced activation of inflammation, creating a precancerous microenvironment – NF-kB, Jun/Ap-1, NFAT, STAT3, & COX2 pathways
c. Inflammation due to other cytokines – Chronic production of pro-inflammatory mediators due to #2 – pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, etc.)
d. Inflammation due to ROS/RNS
e. Inflammation due to senescent cells and the SASP
10. Growth factor overstimulation – growth factors and intracellular pathways involved with proliferation and growth
– EGF, VEGF, HGF, β-catenin, ERK, MAPK, JNK, etc.
11. SASP induced EMT – Precancerous cell transformation to cancer cells by the inducing the epithelial-to-mesenchymal transition (EMT) due to secretions by senescent cells (SASP)
12. Telomere uncapping – Telomere shortening and the resulting genome instability produced by uncapped telomeres
13. Telomerase activation or ALT activation – Activation of telomerase or the ALT pathway in the cancer cells to prevent p53 and p16 induced cell cycle arrest
14. Loss of Contact inhibition – Loss of cell-to-cell contact inhibition, which allows cells to continue to proliferate, despite crowding
15. ECM degradation – increased production of extracellular matrix metalloproteinases (MMPs) that make it possible for tumor cells to invade surrounding tissue
16. Loss of cell cycle control – Loss of cell cycle regulation/control by mutations or epigenetic silencing of cell cycle checkpoints
17. Cytokine-mediated tumor invasion/metastisis – up regulation of specific cytokines or cytokine receptors involved with tumor invasion and metastisis – IL-13, IL-13Rα2
18. Angiogenesis – Activation of angiogenesis by angiogenic growth factors and receptors – VEGF, HGF, TGF-α, TGF-β , PDGF, TNF-α, interleukins, chemokines, and FGF family
19. Hormonal receptor activation – Stimulation of androgen/estrogen receptors by endogenous hormones/metabolites – Androgen receptor (AR), estrogen receptor (ER), etc.
20. DNA repair defects – DNA repair mechanism defects due to mutations or non-mutational epigenetic silencing – BRCA
21. Mitochondrial mutations – Mitochondrial mutations and high ROS production, leading to increased DNA mutation rates
22. Loss of mitochondrial ATP production – loss ability to generate energy from fatty acid oxidation, leading to the cancer cell’s dependence on glucose for ATP production
23. Warburg effect – The induction of aerobic glycolysis (the Warburg effect) where cells are dependent on glucose, have high LDH levels, and this effect promotes metastisis
24. ROS resistance in cancer cells – Expression and up regulation of pathways that protect cancer cells against ROS-induced apoptosis
– Nrf2 pathway/ARE genes, FOXO pathway, etc.
25. Apoptosis resistance – Inhibition of apoptotic factors or overexpression of anti-apoptotic factors –
26. Immune evasion – Defects in anti-tumor immune surveillance – cTGF-β, Toll-like receptors (TLRs), immune cell inhibition (NK, TIL, T, B cells), tumor-derived microvesicles, etc.
27. Cancer stem cells – The existence and selective survival of cancer stem cells in response to chemotherapy and radiation therapy, due to their resistance to apoptosis
28. ARE/FOXO down regulation – Down regulation of anti-oxidant response elements (Nrf1/Keap1 pathway) and FOXO genes for synthesis of endogenous anti-oxidant enzymes
29 Protein aggregate accumulation – Accumulation of protein aggregates due to defects in autophagy, the UPS, improper cleavage of proteins (APP), excessive protein damage by ROS, RNS, “gain of function” mutations, or “loss of function” mutations, and epigenetic dysregulation of genes involved with protein homeostasis
30. Mitochondrial mutations – increasing presence of defective mitochondria damaged by increased levels of ROS => accelerated decline in mitochondria
31. Inadequate Mitophagy – clearance of damaged mitochondria by the mitochondrial-specific form of macroautophagy => further accumulation of defective mitochondria
32. Stem cell decline/dysfunction – Defects in stem cell function, decline in stem cell number, and the suppression of stem cell growth by microenvironment factors (SASP)
33. Cellular senescence – the accumulation of senescent cells, which is a cell that has undergone “cell cycle arrest” but will not die (i.e. “apoptosis resistance”). These cells produce a plethora of harmful cytokines that produce chronic inflammation and stimulate the epithelial-to-mesenchymal transition (EMT), which is required for the transformation of precancerous cells to malignant cells.
34. DNA repair decline – Defective DNA repair mechanisms or declines in the efficiency and accuracy of DNA repair.
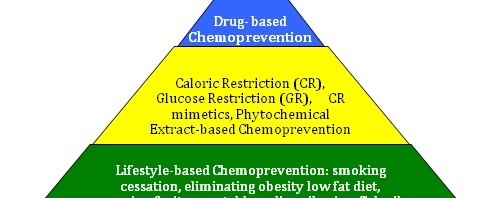
Developing a 3 Tiered Approach for Chemoprevention of Aging and Cancer – The concept of a “Cancer Chemoprevention Pyramid” (CCP)
The pyramid illustrates the timing idea, where the foundation must be always built first. The pyramid also illustrates the magnitude and importance of the base 1st tier, being larger and more important than the middle 2nd tier. The upper 3rd tier likewise is smaller and less important than the second tier. No place is this more important than in cancer prevention, where lifestyle factors have clearly been shown to affect cancer incidence and aging.
The 1st tier strategies are also simple and easy for most people to comply with. The 2nd tier are more difficult to comply with and require much more discipline (for CR and GR), and also require the oral injestion of concentrated extracts obtained from plants and fish. Still, very few negative side effects will occur with both 1st and 2nd tier interventions except for the negative psychological aspects of CR. The 3rd tier of the cancer chemoprevention pyramid is the most controversial, since at this time there are no drugs FDA approved for the prevention of cancer (except for aromatase inhibitors and 5α-reductase inhibitors). Since the FDA does not recognize aging as a disease, no drugs are approved for this indication either. Still, there are over a dozen FDA-approved drugs that have been shown to have an anti-cancer and/or an anti-aging effect. Most of these are well tolerated with few side effects. Many are generic and are inexpensive as well.
The 1st Layer of a Cancer Chemoprevention Pyramid – The “Big 5 Foundation” – eliminating smoking, obesity, and a high fat diet, exercising, and eating a diet rich in fruits, vegetables
.The most scientifically proven way to reduce this risk in both males and females is to stop smoking, which lowers a person’s risk by 8-12%. The next most effective methods to reduce cancer risk is to eliminate obesity, high fat intake, exercise, and eat a diet that is rich in fruits and vegetables. Lifestyle modifications are the “foundation of cancer prevention” and must be implemented or the additional chemoprevention strategies mentioned below will be inadequate to counter the carcinogenic effects of these “big 5” lifestyle factors. With a “Big 5 Foundation” chemoprevention program in place, we believe that a pyramid of additional chemopreventative measures could be implemented that would statistically reduce a person’s lifetime risk of cancer by an additional 30%. There are possible ways of increasing the efficacy of lifestyle modification with the following additional recommendations:
1. Specific “exercise prescription” with specific types of exercise and “doses” – This would include weight lifting with specific goals, aerobic exercise with goals of training within a specified heart rate (i.e. training heart rate), and also adding stretching exercises including yoga. These goals would be gradually increased with time to increase the hormetic dose. This would include heavier weights for weight lifting and more vigorous exercise, based on heart rate during exercise and heart rate recovery after exercise.
2. Specific weight goals based on body fat and lean body mass. For example, this would include a goal of reaching a certain body fat percentage (10-12% for males, 15-20% for females) and certain lean body mass (i.e. building up an adequate muscle mass and maintaining this). The same idea of gradually increasing goals would be instituted here.
3. Specific “superfoods” to be included, such as extra virgin olive oil, red wine, green tea, green coffee beans, curry, soy products, cold water fish, flaxseeds, and lots of fresh berries, fresh vegetables, garlic, onions, and spices (turmeric, cinnamon, allspice). Specific “dose ranges” of these foods with recipes would be encouraged, where a recipe book gave the adequate dosage.
4. Elimination of the Western diet of high fat, red meat, fried food, high calorie foods, sugar-enriched foods, etc.
The 2nd Layer of the Pyramid – Alternate day fasting, calorie or carbohydrate restriction (20g/day), and chemoprevention with phytochemical xenohormetic extracts
The scientific evidence for the beneficial effects of caloric restriction (CR) is now so strong that in 2013, three randomized clinical trials have been approved using this strategy for actually treating cancer (CR for breast cancer treatment, Thomas Jefferson University; CR for prostate cancer treatment, Duke University; CR for pancreatic and lung cancer treatment, U of Iowa). Although it is unpopular, CR has scientifically been shown to reduce cancer risk and increase longevity in all model animal studies. It is time to seriously consider adding a cocktail of natural ingredients extracted from plants to our daily intake, either as an additive to food or drinks, or in a “polypill” form, where 30-50 of the most beneficial herbal products could be combined. One “polypill” could improve the low compliance problem that has been well documented when patients have to swallow dozens of pills per day. The list below includes herbs with a strong anticancer effect. I believe that instituting the 1st layer of the Pyramid and the 2nd layer of the Pyramid could confer a 50% reduction in cancer risk.
| Supplement Source – Name Particular supplement | Xenohormetic Ingredient | Positives and Problems with the particular Supplement |
| Extra virgin olive secoiridoids | decarboxymethyl oleuropein aglycon | Most of the secoiridoids are lost in the waste water from the olive oil presses. It doesn’t get on the shelf |
| Turmeric | curcumin | Best anticancer compound, but there is very low absorption of curcumin and even lower bioavailability |
| Red wine and red grapes | resveratrol | Resveratrol is well absorbed and very bioavailable, but is rapidly excreted by the kidneys in a few hrs |
| White wine and green grapes | n-tyrosol, hydroxytyrosol | White wine is great, but to get enough n-tyrosol and hydroxyl tyrosol, you would have to drink all day |
| Cruciferous vegetables | isothiocyanates (Ex: sulforaphane) | You need to eat a bushel of broccoli, brussel sprounts, cabbage and cauliflower every day |
| Garlic | allyl mercaptan, allicin | Garlic smells. Garlic extracts that do not smell do not have high levels of the active ingredient |
| Onions | quercetin, rutin | Onions smell and make you cry. Extracts that do not make you tear do not have the good ingredients |
| Soybeans, Kudzu | genistein, soyasaponins | Soybeans are very good for many reasons. Unfortunately, genistein also serves as a phytoestrogen |
| Green tea, white tea | EGCG, EGC | Estimates are that you need to drink 15 glasses of green tea per day, to get the proper dose. May be one of the reasons for low cancer risk in Japanese |
| Green coffee beans | caffeic acid and chlorogenic acid | Green coffee beans are good. These polyphenols are damaged by the heat with coffee bean roasting |
| Fish oil, Krill oil | EPA, DHA | There are multiple cardiovascular disease reducable by fish or krill oil |
| Allspice | ericifolin | This spice is one of the best HDAC inhibitors |
| Mushrooms | angiogenesis inhibitors | Mushrooms are great angiogenesis inhibitors |
| Cashew nut shell oil | anacardic acid | Cashew nut shell oil is cheap and is one of the best natural HAT inhibitors and HDAC inhibitors |
| Chocolate | theobromines | Chocolate actually has 3 mTOR inhibitors. Unfortunately, chocolate candy has too much sugar |
| Vitamin D3 | 1,125 dihyroxyvitamin D | Vitamin D3 has been misrepresented as a vitamin. It is actually a hormone and is good for you |
| Vitamin E | α-tocopherol | Only the alpha-tocopherol type of Vitamin E is really good for you. |
| Folic acid | folic acid | This is the best “methyl donor” that you can take. It is important for keeping epigenetics normal |
| Calcium | calcium | Calcium has great anticancer effects. Recent studies suggest that it should be labeled as such |
| Selenium | selenium | Selenium is an element with many isoforms that are beneficial and some that are not |
| Frankincense | boswellic acid | Boswellic acid is only found in minute quantities in plants. Our use of this as a supplement can increase the dose |
| Ginger | zerumbone | Ginger is another very good spice. It is a good HDAC inhibitor. May be one of the reasons for low cancer risk in Japanese |
| Bitter melon extract | charantin, mormordin | Bitter melon extract has some unique compounds that induce apoptosis in cancer cells |
| Cinnamon | cinnamic acid, hydroxycinnamic acid | Cinnamon extracts have all sorts of great features: They are secreted by exosome release. |
| Cinnamon | hydroxycinnamic acid | (-) HDACs |
| Cinnamon | procyanidin oligomers | (-) angiogenesis via VEGR-2 |
| Mangosteen fruit rind | prenylated xanthones and garcinol | Mangostein rinds are one of the best HDAC inhibitors |
| Grapefruit, other sources | naringenin | This is the ingredient that makes grapefruit bitter. |
| Citrus fruits, other sources | Gentisic acid | This is another very good ingredient in citrus fruits with multiple mechanisms of action |
| Red apples, onion | quercetin | Skins of red apples an excellent source. Very good mitochondrial biogenesis polyphenol |
| Piper Longum extract | piperlongumine | This is a polyphenol that increases ROS selectively in cancer cells. It should be exploited for this |
| Tomatoes | lycopene, luteolin, lutein | These polyphenols are mainly in the tomato skins, which is discarded making tomato juice, soup, and ketchup |
| Venus flytrap extract | plumbagin | |
| Red sandalwood oil | pterostilbene, α-santalol | |
| Flaxseed, chia seed | linolinic, linoleic acids | |
| Hot chili peppers | capsaicin | |
| Rosemary, oregano, sage, thyme | rosemarinic acid | |
| Volatile oil of Radix sinensis | n-butylidenephthalide | |
| Triphala churna | chebulinnic acid | |
| Skins from apples, etc. | ursolic acid | This is one of the best polyphenol for addressing skin cancers; it induces apoptosis in such cells |
| Yerba mate | caffeine, theobromine, theophylline | Yerba mate is a good source of all three of these polyphenols |
The 3rd Layer of the Pyramid –Drug Chemoprevention (NSAIDs, COX2 inhibitors, 5-α reductase (-), aromatase (-), SARMs, SERMs, Metformin, Rapamycin, statins, H2-blockers)
There is now a large body of evidence that several over-the-counter medications as well as several classes of prescription drugs can further reduce cancer risk. This includes aspirin, which is the acetylated form of the natural compound, salicylate. Aspirin was once thought to only have an antipyrogen/anti-inflammatory effect via the inhibition of arachidonic acid pathways. We now we know that there are many molecular targets for aspirin, including COX, LOX, AMPK, and mTOR. The use of a FDA- approved drug for a new indication (anti-aging and cancer prevention) is called “drug repurposing”. Here is a list of drugs that could be “repurposed” for cancer chemoprevention and anti-aging. When combined with a lifestyle modification program and a botanical extract “polypill”, I suspect that adding a “polypill” of FDA approved drugs to the plan will result in a 80% reduction in cancer risk.
Drug (or Drug Category) FDA approved indication Anti-aging & Anti-cancer “Drug Repurposing: mechansims of Action
NSAIDs anti-inflammatory (-)Arachidonic cascade, (-)COX, (-) LOX, (+)AMPK, (-)mTOR
Celecoxib arthritis (-) COX3
Metformin diabetes (+)AMPK, (-) mTOR, (+) autophagy,
Rapamycin organ transplant rejection drug (-) mTOR
H2 blockers acid reflux, ulcers (-)angiogenesis, (+) immune stimulation against cancer cells
Statins hypercholesterolemia (-) HMG CoA
5α-reductase inhibitors alopecia, BPH androgen-sensitive prostate cancer prevention
aromatase inhibitors breast cancer breast cancer prevention
orinostat (SAHA) (-) HDACs
valproic acid (-) HATs
JQ1 male contraceptive (-) BET bromodomain protein 4 (BRD4)
Benzodiazepines anxiety, sleep (-) BET bromodomain protein 4 (BRD4)
Lanperisone muscle relaxant selective ROS inducer in cancer cells
Chlorpheniramine maleate allergy drug (-) HDACs
There are some other non-FDA approved compounds that also have a great potential for reducing cancer. Some are considered drugs. Others are considered supplements. Some are non classified of yet. They include the following:
Compound Category of compound Anti-aging and Anti-cancer mechanism of action
Melatonin pineal gland hormone mitochondrial-specific anti-oxidant
Oxaloacetate Citric acid cycle intermediate activates translocation of FOXO to the nucleus
3-Bromopyruvate
2-Dexoyglucose
SARMs
SERMS
MitoQ
PPAR agonists
AICAR
DFMO (diflouromethylornithine)
C60 fullerenes
PQQ
SkQ
Integrating all 3 Tiers of the Prevention Pyramid
| Molecular Solution Strategy for an anti-anti-cancer and anti-aging effect: Lifestyle | 1st Tier of Prevention Pyramid | 2nd Tier of Prevention Pyramid | 3rd Tier of Prevention Pyramid |
| Lifestyle, Food, & Beverage Strategies | Most Effective Natural Product Strategy | Drug Chemoprevention Strategies | |
| 1. HDAC inhibition | caloric restriction (CR), fasting, glucose restriction (GR), green tea, soy products, broccoli, green coffee beans, olive oil, wine, water cress, curry, cinnamon, ginger | EGCG, Genistein, Phenethyl isothiocyanate, curcumin, sulforaphane, reseveratrol, I3C, hydroxycinnamic acid, plumbagin, selenium. Trapoxin A, zerumbone | SAHA, Chlorpheniramine maleate |
| 2. DNMT inhibition | CR, fasting, GR, green tea, white tea, apples, grapefruits, brazil nuts, garlic, raw or rewed tomatoes w/skins, green coffee beans | EGCG, genistein, garcinol, garlic & onion organosulfur compounds, lycopene, lutein, luteolin, chlorogenic acid, caffeic acid | |
| 3. HAT inhibition | CR, GR, fasting, green tea, white tea, curry, soy products | Anacardic acid, EGCG, genistein, curcumin | |
| 4. HMT inhibition | No known 1st tier interventions for this | S-adenosylmethionine (SAM) | BRD4770 |
| 5. Insulin/IGF-1 pathway inhibition | CR, GR, fasting, NO hGH tx, No Insulin tx | Botanical Klotho activators, EVOO secoiridoids | 2-dexoyglucose, metformin |
| 6. mTOR pathway inhibition | caloric restriction, fasting | caffeic acid, EGCG, EVOO secoiridoids, quercetin | rapamycin, metformin, aspirin, sulindac, PI3K inhibitors, Akt inhibitors, MEK inhibitors |
| 7. AMPK activation | exercise, CR, GR, fasting, EVOO | resveratrol, phenethyl isothiocyanate, secoiridoids, oxaloacetate | metformin, aspirin, rosiglitazone |
| 8. Sirtuin activation | exercise, CR, fasting, red wine, EVOO wine, EVOO | resveratrol, myricetin | |
| 9. FOXO activation | CR, fasting, white wine, EVOO | oxaloacetate, leucine supplementation, n-Tyrosol, Hydroxytyrosol | |
| 10. Nrf2 activation | exercise, CR, fasting | isothiocyanates | metformin |
| 11. Ras/Raf pathway inhibition | CR, fasting | No known natural inhibitors of this pathway | B-raf inhibitors – aminoisoquinolone, MEK inhbitors |
| 12. PPARα and PPARγ activation | exercise, CR, fasting | oleoylethanolamide, phytannic acid, PQQ | fibrate, fenofibrate, ciglitazone, rosiglitazone |
| 13. NF-kB inhibition | exercise, CR, GR, fasting, curry, green tea, green coffee beans, Venus fly trap extract | polyphenols (curcumin, EGCG, isothiocyanates, caffeic acid, chlorogenic acid, etc.), plumbagin | |
| 14. AP-1 inhibition or activation | exercise, CR, GR, fasting, curry, green tea, white tea, broccoli, brussel sprouts, cabbage, watercress | polyphenols (curcumin, EGCG, isothiocyanates, etc.), plumbagin | |
| 15. JAK and STAT3 pathway inhibition | exercise, CR, GR, fasting, wine | curcumin, resveratrol, cucurbitacin derivatives, flavopiridol, deoxytetrangomycin, piceatannol,indirubin,plumbagin | |
| 16. Arachidonic acid/COX2 pathway (-) | No lifestyle interventions for this | Fish oil, Krill oil, myriad algae, curcumin | NSAIDs, COX2 inhibitors |
| 17. Heat shock protein activation | repeated mild heat stress (RMHS), repeated mild hypoxic stress | secoiridoids | |
| 18. Retinoic acid receptor activation | No lifestyle interventions for this | naturally occuring retinoids | all-trans retinoic acid, 13-cis retinoic acid, bexarotene, fenritinide, |
| 19. β-catenin signaling inhibition | No lifestyle interventions for this | fisetin | JQ1, iBET 151, triazolo-benzodiazepine |
| 20. c-Myc inhibition | No lifestyle interventions for this | no naturally occuring c-Myc inhibitors | |
| 21. VEGF/VEGFR inhibition | No lifestyle interventions for this | Vit E, fish oil, H2-blockers, mushrooms, curcumin, garlic, emodin, coleon A lactone, gentisic acid, rapamycin | many drugs for angiogenesis inhibition |
| 22. EGF/EGFR inhibition | No lifestyle interventions for this | ||
| 23. SKP2 inhibition (part of UPS that degrades tumor suppressor proteins) | caloric restriction, fasting | gallic acid, EGCG, quercetin, curcumin, lycopene, secoiridoids | no drugs for Skp2 inhibition |
| 24. Statins | Lovastatin, others | ||
| 25. Warburg effect inhibition (LDHA gene) | No lifestyle interventions for this | secoiridoid polyphenols | metabolic disruptors – 2-Deoxyglucose, MitoQ, |
| 26. Inhibition of cellular senescence | No lifestyle interventions for this | secoiridoid polyphenols | |
| 27. Inducing ROS or increasing ROS induced apoptosis | hypoxia can augment chemotherapy-induced apoptosis; heat can augment chemotherapy-induced apoptosis |
Wrapping it up There are a great many similarities in the epigenetics and biological processes of cancer and aging.
- Therefore, it appears that if effective means could be found to prevent cancer, those means would most likely also be preventative of aging itself.
- The impact of widespread adoption of such means could have significant impacts on extending average human lifespans, both because of less deaths by cancer and because of the reduced rates of aging.
- So, we have been concerned here with how common strategies might be found that address both cancer and aging.
- A key concept to bring to bear on this issue is the process of Xenohormesis,whereby stress-response phytochemicals developed over millions of years of evolution in plants keep plants cancer-free and healthy appear to be capable of doing the same in humans.
- We have herein provided first-level molecular explanations for the “causality” of cancer and aging. We have described the processes in cancer and aging of epigenetic silencing of “good” genes and epigenetic activation of “bad” genes.
- Further, we describe how these unwanted epigenetic effects can be reversed by judicious ingestion of plant-based phytochemicals.
- We identify a 3 tiered “Pyramid” approach for chemoprevention of aging and cancer, the exact interventions involved in each layer of the Pyramid, and how the interventions in the three layers of the Pyramid can be integrated together.
DATA DISCLAIMER:
The tables and compilations of data in this and the other blog entries in the Two Dragons series are intended to be illustrative of the main points to be made. They are compiled from various sources, in most cases are incomplete, and may contain occasional errors.
MEDICAL DISCLAIMER
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S SPECIFIC MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION





Hi Vincent,
I have been reading your blog for quite a while and am fascinated with much of what you have to say. One concern I do have is about the preference for low fat diet. Much of what I have been reading on other health blogs claim that I should be eating more fat (butter, coconut oil, etc) while lowering starch levels (Daf16 up-regulation and ketone triggered autophagy) while cycling protein(autophagy). As you can guess this gets very confusing. Some blogs I read state saturated fat is fine if from a healthy source and to limit omega 6 fats (or at least balance with omega 3). Do you have any recomendations or perhaps somewhere to point me to on the dangers of fat?
Thanks again, and as always I loved the post.
Hi Shanehen
Thanks for your comments.
While there is conventional wisdom favoring a low-fat diet, I personally do not believe that all fats are bad, including saturated fats. Rather, I believe that sugars and empty carbohydrates should be avoided. There is a fairly good argument for tilting diets in the ketogenic direction and I personally consume a certain amount of coconut oil. This blog entry was not focused on dietary components and only mentioned the low-fat idea as incidental. I will probably generate a blog entry related to cattle generate diets in the future. Finally, I quite agree that a very key matter in all of this is maintaining an adequate omega-3 to Omega-6 six ratio.
Vince
This was a great overview, but it is superficial on several conventional topics.
High Fat: Is not unhealthy per se. What is unhealthy is high intakes of Omega-6 fats which are oxidized during digestion (if not also beforehand during processing, as Omega-6 is inherently unstable), at least past the small hormetic dose required (around 11g/day). Oxidized Omega-6 is easily incorporated by LDL into its cell membrane and thus becomes oxidized LDL. So stable saturated and monounsaturated fats are preferred and needed by the body for optimal helath. We already banned trans-fats in the food chain for the large part, so next up is getting rid of Omega-6 which is the mother precursor to those trans-fats.
Hormone Replacement: This is conflating xenobiotic hormones from animals with bioidentical (naturally human) hormones. High hormone levels are actually protective against cancer, otherwise everyone under 25-30 would have high cancer rates. That doesn’t happen. The “hormone causes cancer” hallucinotion was conjecture from a single source way back 50-60 years ago and Harvard Medical finally put it to bed for good a few years back. As for HGH, its only causes problems when doses used are past the hormetic, which is indeed responsible for all the negative press or sloppy research about it.
Red Meat: Pork seems to be a possible new culprit (which is also the most commonly form of processed meats), not ruminants. But the evidence is unconvincing so far to delineate. Still better to donate blood regularly to reduce the iron factor.
Xenohormesis: Far overlooked is the role of anti-nutrients and toxins in carb-source plants such as grains, legumes, nuts and seeds. I would expect people eating such regularly to get higher doses relative to polyphenols, causing an almost endless variety of health problems from affecting the gut microbiome and instestinal permeability at the very least. This bleeding edge, vanguard research right now.
Glucose Restriction: The body has a hormetic need for 480-600 calories of glucose (120-150 grams) a day before oversaturation. Levels below that inversely cause more and more serious health problems, especially at radically extreme levels such as 20g/day. The same hormesis logic holds true for protein at 300-600 calories, but in the battle between protein and glucose, glucose will win out as it is protein sparing, thus requiring less protein needed to preserve muscles. Excess protein also shortens lifespan from overexposure to methionine at the very least. So, say you limit your glucose and protein to the hormetic range and limit your overall calorie intake for life extension… how the heck do you then get enough calories without eating an “unhealthy” high fat diet? D’oh! See above.
Machine Ghost
Wow. First of all, it is really good to see that people are reading these blog entries and are willing to weigh in with intelligent comments. With regard to the importance of the omega-3 omega-6 ratio I have already commented that maintaining an adequate levels of omega-3 is essential to health. The blog entry was really not about high fat or low-fat or base level foods in the Pyramid. Rather it was about the epigenetics of cancer and aging and how phyto substances and certain chemical substances could be used to rectify age-related epigenetic aberrations.
I tend to agree with you regarding the issues of red meat being primarily associated with industrial-lot maintained animals and possibly existing less with ruminant animals. Again this was not the focus of the blog entry.
With regard to anti-nutrients and Xenohormesis, you make a very good point. There is possibly a significant difference between the positive xenohormetic impacts of natural plant polyphenols which were derived as stress responses and the possibly negative xenohormetic impacts of grains that have carefully been bred by humans for matters like insect resistance, storage without spoiling, etc. While I very much agree with you with regard to the presence of anti-nutrients, I’m not familiar with research that establishes xenohormetic actions. If you could point me to publications specifically relating to such xenohormetic impacts I would appreciate that. I would be particularly interested in xenohormetic impacts that negatively affect the gut biome.
With regard to your final point on Glucose Restriction it seems you have an excellent “goccha” there. Eat fat!
Primary author Jim Watson, do you want to weigh in on some of these dietary points?
Vince
In general, I agree with the above two replies – I too find it hard to reconcile the obsession with low fat diets when there are multiple studies proving that higher fat diets are not problematic or detrimental to health. In fact in many cases, the opposite is proven to be true. As stated in the above replies – it is the nature of the consumed lipid and the quantities consumed that is important. The Western diet in general, has a huge discord between omega 6 and omega 3 intake, which is a huge contributing factor to long-term, low grade inflammation at the cellular level. Once it was thought that cancer, metabolic syndrome etc, was the cause of inflammation processes involved in these conditions, however a number of researchers now think it is the long-term, low grade inflammation that, over time, largely stimulates the disease process – not the other way around!
I eat a high fat, moderate protein and lower carbohydrate diet and have done so for 2.5+ years. My bio-chemical blood results are all perfect and my Doctor refuses to repeat them – he says testing me is, “A waste of time and money.” I feel 100% better than when I ate a high carbohydrate diet as suggested by dieticians. In time, maybe I will find my present diet is detrimental, but at present, the way I feel (and look – I am 47 yo, with sub-10% body fat and good muscle mass), I don’t think I am wrong – but time will tell. I eperimented with multiple diets, macro nutrient proportions etc. and found, after much trial and error, the above diet to be best.
As stated by MachineGhost, grains and legumes in particular contain multiple anti-nutrients such as phytic acid and oxalates, which bind to digestive enzymes, reducing ones ability to absorb and assimilate nutrients consumed from all foods.
This diet may not suit everyone, but it does seem to suit me. I feel very ill if I am out with other people and have the choice of predominantly grain-based foods to eat, although, as I have got leaner, my body seems to cope with them a bit better than before. I could never return to those foods as being the mainstay of my diet – eating like that made me very unhealthy.
Electhor
Please see my comments above in response to Machine Ghost and shanehen. I find myself pretty much in agreement with everything you say and share your doubts about the healthfulness of many of our mainstay grains which have been bred for properties related to industrial production, in some cases resulting in detrimental health impacts.
As to your own diet it is great that you have found something that works well for you. Your comment as well as Machine Ghost’s and Shanehen’s comment point out how the conventional wisdom at the bottom level of the food pyramid may need serious revision.
Vince
Vince,
you say that “α-tocopherol” is “Only the alpha-tocopherol type of Vitamin E is really good for you.”.
I thought that α-tocopherol depleted gamma-tocopherol, and that tocotrienols were the best form. I’m now puzzled.
Best. Santiago
Hi Vince,
I did not see your reply to MachineGhost. Although I posted after you, I started writing before you posted and due to intrruptions took a while to finish. I did not check whether there were ‘new’ replies before I posted..Sorry.
Any way, I relaise that high fat, low fat was not the main aim of the blog, however, I, like the first two people, did see it as one of the main points mentioned in the ‘first tier’ of the pyramind, it thus ‘stuck out like a sore thumb’, when there is much information contrary to that view.
When I got interested in this subject, I had in the back of my mind exactly the same premise as this blog, ie that consumption of phytonutrients, correct diet/exercise etc. should have the effect of fighting/preventing/delaying cancer and aging at the same time! Jim has put the material together much more eloquently than I could. He has also expanded somewhat on my ‘simplified version.’
I however have issue with the dietary guidelines as set out in the first tier of the pyramid – it reminds me of the guidelines popularised 30-40 years ago, which are at best questionable in their ongoing relevance and most likely, not true…reminds me of the ‘oxidative theory of aging’, which is now dead in the water, untrue etc. but remains a ‘true theory’ in peoples psyche’s, the media and advertising.
[As a side note; a review of the food pyramid is long over due – it’s relevance was first questioned in about 1929!]
It was also refreshing to see someone finally write that calorie restriction (CR) does not seem to extend life-span in primates. There are two long-term studies completed in the past year that both came to the same conclusion, but most information one finds on CR, continues to make claims about the great life extending benefits it produces. In lower animals it seems to, but not in primates and thus humans!
Great article again, I’ve been doing a bit of reading through the journal articles on epigenetic lately, esp in regards to the Nrf2/glutathione system, and then I get this gem to read through!
“Curcumin – Best anticancer compound, but there is very low absorption of curcumin and even lower bioavailability”
Have you looked into the Longvidia curcumin?
http://www.longvida.com/index.php
At little bit more pricey as always, but the pharmacokinetic studies look good.
“This clinical data is consistent with human pharmacokinetics (bioavailability) data, where Longvida was 65 times more bioavailable than unformulated curcumin based on Cmax, and more than 100 times more bioavailable based on Area Under the Curve (AUC). One groundbreaking finding over the course of several bioavailability studies using different doses, is that dosing of Longvida leads to therapeutic levels of free (not inactivated or glucuronidated) curcumin in the bloodstream and target tissues.”
For the isothiocyanates (sulforaphane) the best way other than supplement form is broccoli sprouts, which have much higher concentrations than the full grown veggies.
I’ve noticed a number of studies where there is synergistic effects between the various phytochemicals.
And good to see we’re all in agreement that a diet high in healthy fats is good. Low carb and low fat starts to cut out the options pretty quick!
electhor:
I certainly agree with you about Jim Watson’s brilliance and ability to put relevant research into a comprehensive framework. I feel very fortunate to have him as a close colleague and contributor to this blog. We tend to spend a lot of time communicating and it is worth every second.
And yes, the food pyramid did remind me of the publications in popular magazines of 40 years ago on how to eat well. I am a bit skeptical about it and prefer to be concerned mainly with the second tier of the pyramid.
Vince
Dan Campagnoli:
Thanks for your comments Dan. With regard to the Nrf2 pathway, you probably know that I have posted a lot of blog materials regarding this. If you are not familiar with them just do a search for blog entries having to do with Nrf2. It is a very interesting pathway in part because substances that activate it tend also to inhibit the NF-kappaB pathway and runaway inflammation.
As far as curcumin is concerned, I agree that there is a major issue of bio availability, and that in some medical studies truly therapeutic doses are difficult to achieve without side effects. Up to this point I have not looked into the Longvida supplement although I have been personally using another high – availability supplement Curamed. I will compare the two because I agree that, of all the phyto substances, curcumin is very particularly important. It, and then probably resveratrol.
I quite agree that the phyto substances behave rather differently, both in their epigenetic’s and other impacts, and that probably it is a excellent idea to supplement with a number of them.
Good hint, about eating broccoli sprouts. You may know that a few colleagues and I have formed a startup consulting company concerned with the use of the phyto substances for creating health http://www.vivaceassociates.com.
Vince
Hi Vincent,
I finally got around to having a look into the Curamed curcumin you take.
When I was looking up different different curcumin formulations available I had suggested the Longivida from this write up, he does a good job comparing the studies of a few curcumin variants which you should find interesting
http://truttmd.com/curcumin-caveat-emptor-not-all-brands-are-created-equal/
It’s looks great what you have started with Vivace. I have an interest/goal in being involved with something like that down the track to put into practice my interest and knowledge in this area. At the moment I have an involvement with a company with a very effective compound that fits into the redox/hormesis space which I think you will find interesting. Also I’ve been writing up a related article which ties right in with Nrf2 and my submission on the wager of the most important factor 🙂
I had another idea too to help spread all the great information here on your blog to suggest to you, will have to get in touch via email.
Daniel
Pingback: PART 2: Slaying Two Dragons with One Hail of Stones: The Silencing Of Good Genes In Aging And Cancer – And How Polyphenols Can Prevent That | AGING SCIENCES – Anti-Aging Firewalls
Pingback: PART 3: Slaying Two Dragons with the Sound of Silence: – How to Keep Your Repetitive DNA Turned Off with “3 Songs”: Sirtuins, Polycomb Proteins, and DNMT3. And a Master List of Drugs and Natural Compounds for Cancer Chemoprevention | AGING SCIEN
Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum -http://www.ncbi.nlm.nih.gov/pubmed/22692848
Also known as “kelp”.
“A. nodosum extract 0.2 % stimulated SIRT1 activation. When it was incubated for 20 min, A. nodosum extract induced a 1.65-fold change in SIRT1 activity, and when it was incubated for 24 h, it induced a 2.33-fold change in SIRT1 activity (Fig. 4). Resveratrol was used as a positive control for SIRT1 activity.”
http://link.springer.com/static-content/images/335/art%253A10.1007%252Fs12010-012-9761-1/MediaObjects/12010_2012_9761_Fig4_HTML.gif
“The tested A. nodosum extract, rich in phlorotannins, showed potent antioxidant, anti-inflammatory, and pro-SIRT1 activities. Such an extract, able to prevent ROS production and cytokine release and to stimulate SIRT1, is very promising in antiaging therapies.”
“the 6 groups of genes that if silenced, the cell can form cancer without any DNA mutations”
Are you suggesting that this isn’t just an element of cancer progression, but that a purely epigenetic cancer could exist? With no DNA mutations at all? This has occurred to me but I haven’t come across evidence that it exists, or that if it did exist that it would be defined as a cancer at all.
1) Vince, I’ve seen your site a while back, but, regrettably, haven’t read much from it. This is a gold mine, though. It’s my goal to find the time and read all of your posts for the past 2 years.
2) I vehemently disagree with all the high fat people. I will try to post my opinions on the matter when I find the time. I will save that battle for another day.
3) I started working on my own regimen a few months back. This is a work in progress, to say the least. This regimen is tailored to me and my issues. http://selfhacked.com/2013/06/10/my-current-regimen/
I was thinking that it would be cool if we can have a document on google docs, where there would be a group collaboration -a Wikipedia type article -detailing important diseases and mechanisms behind these diseases and plant substances which modulate these mechanisms, with all the references. You and I have made some form of such documents. A collaboration like this with the world will greatly enhances everyone’s knowledge.
Keep up the great work!
Pingback: Quorum sensing Part 1: quorum sensing inhibition via phytochemicals – a new approach against infectious diseases. | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Health through stressing fruits and vegetables – the Xenohormetic Live Food Hypothesis | AGING SCIENCES – Anti-Aging Firewalls
Pingback: What do we need to do to live longer, healthier lives? An editorial tale of cars and people | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Inflammation Part 2: The Tale of Three Stress Sensors and their Interactions: 1)Inflammation, 2)Genomic Instability (p53), and 3)Oxidative stress (Nrf2) - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™