by James P Watson and Vince Giuliano
There is a community of self-experimenters who are taking the drug Metformin, not because they are diabetic or prediabetic, the FDA-approved reasons for prescribing the drug, but rather because they believe it probably has an impact in promoting general health and retarding aging(ref). In fact, it is probably the pharmaceutical most used for this purpose. The purpose of this blog entry is to discuss Metformin as it has been shown to reduce all cause mortality in various studies, and discuss its understood mechanisms of operation.
This blog entry is Part 2 of interventions that reduce all cause mortality. The first blog entry in this series, which was also Part 4 of the inflammation series. Was concerned with PCSK9 inhibition.
What Is Metformin?
Metformin is a traditional and inexpensive drug that is the first line of treatment for people with Type 2 Diabetes or prediabetic conditions. It is a drug that has been extensively studied, both with respect to the impacts of using it and as to the molecular mechanisms of its operations.

Image source Metformin “originates from the French lilac or goat’s rue (Galega officinalis), a plant used in folk medicine for several centuries.[114] ”
“Metformin has been used for over 40 years as an effective glucose-lowering agent in type 2 DM. Typically it reduces both basal and post-prandial hyperglycaemia by about 25-30% on over 90% of type 2 DM patients when given either alone or in combination with other therapies —”(ref)
Clinical trials of Metformin
Clinical trials.gov lists 1989 clinical trials mentioning Metformin but only three clinical trials mentioning Metformin and longevity. All three of these are placebo controlled. One is called the Metformin in Longevity Study (MILES)sponsored by the Albert Einstein College of Medicine is currently listed as active but not recruiting and is the only study where aging is the unique endpoint condition. A second study is concerned with Metformin and Longevity Genes In Pre-Diabetics where the conditions being tested for our insulin resistance aging and inflammation. This study is listed as completed. A third study is concerned with Metformin And Longevity as related to with patients with prostate cancer. Its current status is unknown.
The TAME trial
The American Federation of Aging Research is sponsoring a clinical trial of the impact of consuming Metformin on a general population on aging, TAME standing for Targeting Aging With Metformin. A number of prominent aging researchers, particularly Nir Barzilai are backing the trial and it has received widespread mainstream media publicity. We do not know the relationship between the TAME trial and the MILES trial mentioned above.
The TAME trial has been approved by the FDA and will seek to follow two populations, one of 1500 people who will receive metformin, and an equal size control population who will receive a placebo. The trial will look at whether Metformin delays the onset of aging–related diseases or disease precursor conditions such as cancer, cardiovascular diseases and Alzheimer’s disease, and of course ACM. This study will include people 70 to 80 years old in locations across the United States. They will be followed for 5 to 7 years. Participants will be included who may have or be at risk for certain diseases such as cancer, heart disease, or cognitive impairment. The study will not include Type 2 diabetics because it is intended to measure the anti-aging effects of Metformin apart from its known capability to significantly reduce ACM in diabetics..
The TAME trial was reportedly first discussed with the FDA in 2014. To our knowledge, however, now in 2017 the trial is yet to be funded. If the TAME study is funded and when its results are known many years from now, we should have trustworthy statistics related to the longevity impact of normal healthy people taking Metformin. At present, the best surrogate measure for the longevity impact of consuming Metformin is ACM. Unfortunately, the clinical trials that produce the statistics were based on populations of diabetic people. Knowing the molecular mechanisms involved in Metformin’s activities. we assume that there is also a longevity impact on people in the general population, but we do not know what that impact is,
Metformin – 24-36% reduction in All Cause Mortality, 40-57% reduction in cancer mortality
Metformin used to be in 1st place on our list of drugs for all-cause mortality reduction, but we knocked it down several notches as a result of reviewing the recent data on fish oils, PCSK9 inhibitors (see our recent blog entry on them}, testosterone, and SGLT2 inhibitors.
One of the best studies of Metformin vs other more conventional therapies for type II diabetes was done in Britain and was called the United Kingdom Prospective Diabetes Study (UKPDS). This was a 20-year prospective study of over 5,000 patients treated with either Metformin or other conventional therapy (sulphonylurea or insulin). Here is the data on how much Metformin reduced diabetes-related deaths, all-cause mortality, myocardial infarction, and microvascular diabetic disease.
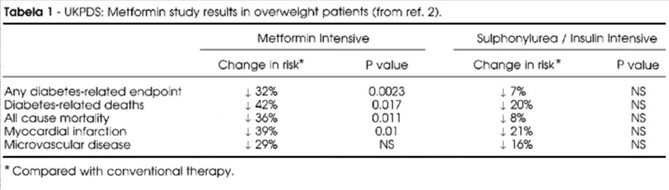
Reference and table source: Metformin and The United Kingdom Prospective Diabetes Study: A Commentary (2000)
This ACM reduction is quite astounding! Not all studies show such a dramatic effect, however. A reference below is a meta-analysis that casts doubt on the value of metformin in reducing all-cause mortality, even in diabetics. However, the initial results of the UKPDS study were so dramatic and a long term follow-up article showed that the reductions in cardiovascular mortality (15%) and call-cause mortality (13%) in the UKPDS trial persisted for 18 years, although the “size effect” of the reductions was smaller.
Will dramatic beneficial effects of Metformin be seen in nondiabetics who are put on metformin for “anti-aging” purposes or will we see side effect that outweigh the benefits? Only when Dr. Nir Bazali’s TAME trial is funded and the many years from now when the study is completed will we have an answer to that question. In diabetics, Metformin monotherapy vs Sulfonylurea therapy shows a 58% higher all-cause mortality in those on sulfonylureas. In older men with prostate CA and diabetes, Metformin users had a 24% ACMR in the 1st 6 months of use and a reduction in prostate cancer-specific mortality of 24% as well (HR = 0.76). However, this effect waned over time.
Metformin may reduce death by several causes. Meta-analysis of papers on cancer and Metformin show a 57% reduction in cancer incidence and cancer mortality in diabetic patients on Metformin vs other diabetic drugs (OR = 0.43)(Landman, et.al., Diabetes Care). Thus the ACMR probably is due to more than just diabetes deaths. Insulin therapy, on the other hand, dramatically increases the risk of cancer in diabetics by 69% to 363%! (No surprise!).
How does Metformin work?
Here are some diagrams that help illustrate that:
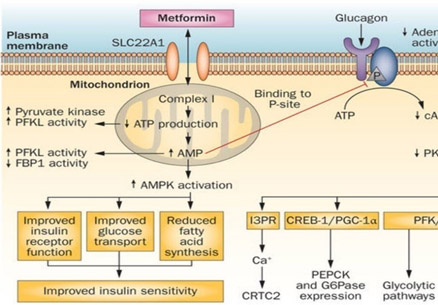
Reference and image source: Metformin—mode of action and clinical implications for diabetes and cancer (2014)
Anti-diabetic Molecular Mechanisms of Metformin
The above diagram illustrates how Metformin enters the cell by active transport systems, such as the SLC22A1 transporter. Once it is inside the cell, it migrates to complex I in the mitochondria where it inhibits ATP synthesis. As a result, the cell thinks it is in “energy bankruptcy”, effectively activating all of the caloric restriction pathways. The most direct CR pathway activated by Complex I inhibition and ATP synthesis blockade is AMPK. AMPK is the enzyme that you activate when you exercise. ATP depletion and the resultant increase in AMP levels drives AMPK to improve insulin receptors on the cell surface, improve glucose transport, and reduce fatty acid synthesis. This results in improved insulin sensitivity. AMPK also inhibits glucagon signaling, however. This results in a reduction in all of the downstream effects of PKA enzyme activity, which include glycolytic pathways and gluconeogenic pathways. However the above diagram fails to illustrate many additional molecular mechanisms of Metformin. These include cancer chemoprevention pathways such as the ones diagramed below:
Cancer Chemopreventative Molecular Mechanisms of Metformin
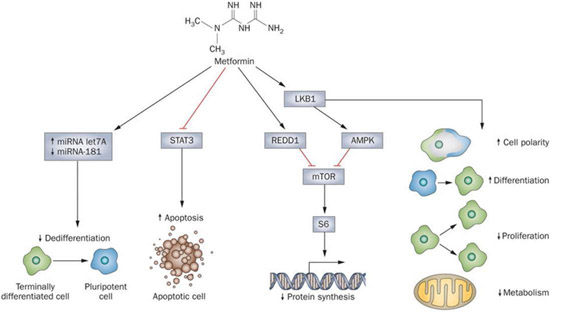
Reference and image source: Beyond aspirin—cancer prevention with statins, metformin and bisphosphonates (2013)
The 4 Major “Anti-Cancer” Molecular Mechanisms of Metformin – microRNA, STAT3, mTOR, AMPK
In the diagram above, the illustrators try to show how Metformin reduces cancer risk by 30-50%.
The 4 major mechanisms for this include 1. increases in miRNA Let7A and decreases in miRNA-181, which lead to reduced “dedifferentiation” of terminally differentiated cells into pluripotent “cancer stem cells”; 2. the inhibition of the STAT3 pathway, which is involved with apoptosis and cellular senescence; 3.the activation of REDD1 and 4. LKB1, which activates AMPK and inhibits mTOR. This results in decreased protein synthesis and increased autophagy. Let-7 is a key microRNA that is activated by KSRP, which is a downstream target of AMPK. LKB1 also increases cell polarity, cell differentiation, and reduces cell proliferation and metabolism. All of these mechanisms may play a role in the dramatic reduction in cancer risk seen with Metformin use. However there may be yet-more mechanisms of how Metformin works, other than the anti-diabetic effects and these anti-cancer effects. Many believe there is possibly a more direct way that Metformin slows down epigenetic aging involving DNA methylation. This mechanism is described in the next section.
References:
10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes (2008)
Metformin and The United Kingdom Prospective Diabetes Study: A Commentary (2000)
Here is a list of publications that discuss the use of Metformin along with statins and other pharmaceutical off-label for the prevention or treatment of cancers.
Metformin Alters Genome-wide DNA Methylation – Metformin can slow epigenetic aging
Until recently, all attention on Metformin was focused on its effects on glucose metabolism, mitochondrial complex I inhibition, AMPK activation, and the 4 anti-cancer mechanisms (Let-7, miR-181, STAT3, REDD1/mTOR, LKB1/AMPK). However just recently this year, an entirely new molecular mechanism was proposed by Zhong and colleagues from Yale University. They showed in a very elegant study that Metformin induces genome-wide changes in DNA methylation by modulating the activity of S-adenosylhomocysteine hydrolase (SAHH), the enzyme that degrades S-adenosylhomocysteine (SAH). As it turns out, SAH is a molecular intermediate in the methionine cycle and is the byproduct of DNA methyltransferase transferring a methyl group from methionine to DNA cytosines. If the methionine cycle slows down due to homocysteine build-up or if SAH levels build up for other reasons, DNMT3B is directly inhibited by feedback inhibition by SAH. DNMT3B is one of the major de novo DNA methylation enzymes and the only one of the 3 DNMTs that increases with aging. Here is a diagram of the effect of SAH on DNMT3B.
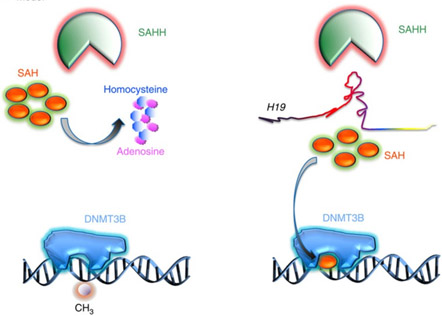
Image source: H19 lncRNA alters DNA methylation genome wide by regulating S-adenosylhomocysteine hydrolase (2015)
SAHH is the only enzyme in human cells that can degrade SAH. Unfortunately, the SAHH enzyme is inhibited by a long noncoding RNA called “H19”. As it turns out, H19 is normally degraded by a microRNA called Let-7. Metformin upregulates Let-7 by an AMPK/KSRP-mediated molecular mechanism (already described in the previous section). As a consequence, Let-7 can trigger the degradation of H19 which then allows SAHH to get rid of the SAH build-up. The net effect is that DNMT3B is no longer inhibited and can resume normal genome-wide DNA methylation. Here is a diagram and the reference for this study:
This pathway too is thought to contribute to the anti-cancer as well as anti-aging properties of Metformin.
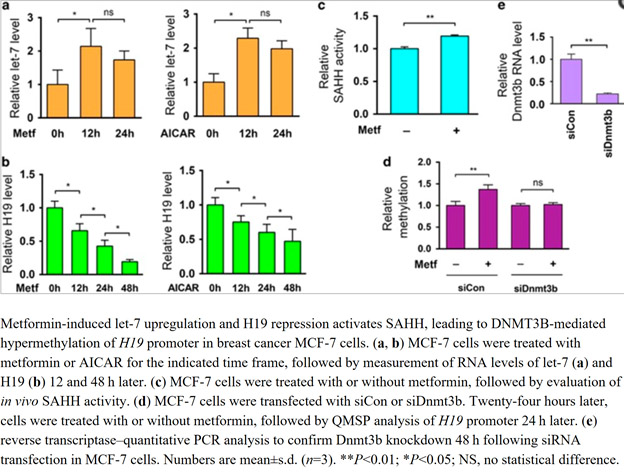
Image and legend source: Metformin alters DNA methylation genome-wide via the H19/SAHH axis (2017)
Additional references:
S-adenosylhomocysteine hydrolase downregulation contributes to tumorigenesis (2008)
Dosage, Side Effects, Cost, and Cost Efficacy of Metformin Therapy
one of the purposes of this blog is to provide a practical resource that you can personally use on your journey to improved healthspan. It is not meant to be a substitute for your own personal physician. Vince and I also do not recommend that you “play doctor” and treat yourself. While we both advocate self-experimentation, we cannot recommend this as a substitute for a licensed health care provider who can be much more objective about our health that we can. With that in mind, here is some possibly useful information about Metformin, dosage, side effects, cost, cost efficacy, and alternatives.
Formulations – Metformin comes in regular forms in doses of 500 mg, 750 mg,850 mg, and 1,000 mg. It also comes in an extended release form in doses of 500mg and 1,000 mg that last much longer.
Daily dosage – For diabetes, Metformin doses are typical starting doses are 500 mg twice a day or 850 once a day, then increased as needed to lower HgA1c. For the extended release forms, typical starting doses are 500mg or 1,000 mg once per day and increased slowly, based on patient’s HgA1c.
Side effects – Lactic acidosis is a rare but serious complication of Metformin therapy that can kill you. It occurs most often in renal failure patients, in dehydrated states, with excessive alcohol intake, and in sepsis. It most commonly occurs with plasma levels of metformin are greater than 5 mcg/ml.
Reference: Metformin dosage guide from Drugs.com
Cost – Metformin is generic, so the cost is very reasonable. The cash price for 14 tablets of 500 mg Metformin is about $10, although much cheaper prices can be found online, especially from Canada pharmacies.
Cost effectiveness – Metformin is very cost effective, since it is generic and has been generic for a long time. When compared to lifestyle intervention for treating T2D, Metformin is either as cost effective as lifestyle programs or more cost effective, provided that the person complies with lifestyle modification.
Reference: The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention (2012)
Alternatives – For those who prefer a natural product over Metformin, there is a good natural product that has a very similar mechanism of action called Berberine. Berberine inhibits gluconeogenesis, inhibits mitochondrial complex I, activates AMPK, and inhibits mTOR. It is unclear whether if exercises impacts through the other channels of operation of metformin described above, such as related to cancer, methylation or longevity. It does appear, however, that this alternative has one other mechanism of action that the drugstore may not even know about – that is the activation of PPAR-gamma. For those who prefer an AMPK-promoting synthetic compound, the synthetic compound called AICAR has shown similar results. AICAR is very effective as an “exercise mimetic”, if such a thing exists.
Conclusions on Metformin
More and more molecular mechanisms are being discovered that may ultimately explain the many pleotropic effects of Metformin. The anti-diabetic effects are mostly mediated by mitochondrial inhibition of Complex I in the electron transport chain. This leads to cellular ATP depletion, which then activates AMPK. AMPK activation induces a host of downstream effects that improve insulin sensitivity and inhibit glucagon signaling. The anti-cancer effects of metformin may be more diverse and include miRNA-181, Let-7, STAT3, REDD4/mTOR, and LKB1/AMPK. However there is a brand new, more recently discovered molecular mechanism called the Let-7/H19/SAHH/DNMT3B pathway. This probably explains the longevity effects of Metformin, which cannot be fully explained by the molecule’s effect on AMPK, mTOR, and glucose metabolism genes. Metformin is cheap, cost effective, with few side effects except for lactic acidosis. It is usually the first line of therapy for type 2 diabetes mellitus.
——————————————
We expect to publish several more blog entries in this series on interventions that reduce all cause mortality. The next one, currently in the works, will probably be the Fish Oil Story
MEDICAL DISCLAIMER
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION.


Pingback: Inflammation Part 4 - PCSK9 inhibition - Also Part 1 of interventions that reduce all cause mortality (ACM) - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™