By Vince Giuliano and Steve Buss
It is now generally accepted that the brain inhibits inflammation induced by an immune challenge resulting in the release of inflammatory cytokines or TNF in two main ways: biochemically, by activating the hypothalamic-pituitary-adrenal axis to release glucocorticoids; and neurally, via a mechanism that has been termed the ‘inflammatory reflex’. This blog entry is about the later.
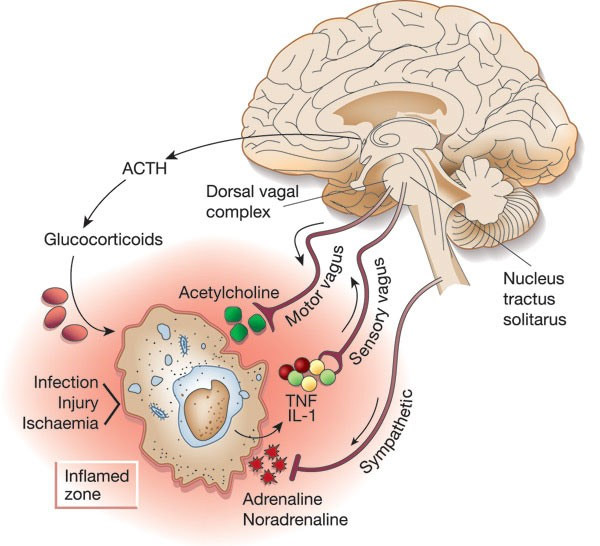
The Inflammatory Reflex (IR) is an autonomous nervous system mechanism for detecting and correcting conditions of inflammation, It is an ancient evolutionarily preserved response found in mammals and important for us. In fact, neural regulation of immune cell activation is an ancient mechanism dating back to nematode worms, IR is a whole-body response that takes place unconsciously in the background via the afferent (incoming) and efferent (outgoing) branches of the autonomous nervous system. Most other causes of and responses to inflammation discussed in this blog and in the literature have been based on cell-level molecular interactions or purely molecular signaling. My colleagues and I have focused so far on matters like NF-kb and other inflammatory and anti-inflammatory cytokines and inflammasomes, and mainly on activities that go on within individual cells. By contrast the IR is a calculated intercellular brain-driven response. It utilizes long-distance electrical links that work in concert with chemical messaging. It is a mechanism coordinating the nervous and immune systems that explains key aspects of the mind-body connection. It opens the door to basically new forms of preventative and active therapies for multiple inflammation-related conditions, such as via electric stimulation of the vagus nerve. Finally, and fascinatingly, it appears that a requirement for the IR to function is the presence of JDJM3, the key histone deacetylase substance that activates the YOUNGING1 pathway that we have written so much about recently. It now appears to us that JDJM3 activation is required for ALL key biological renewal pathways in us. (“A substance (or ligand) is cholinergic if it is capable of producing, altering, or releasing acetylcholine, or butyrylcholine (“indirect-acting”), or mimicking their behaviors at one or more of the body’s acetylcholine receptor (“direct-acting”) or butyrylcholine receptor types (“direct-acting”)(ref).”
THE INFLAMMATORY REFLEX AND SURVIVAL PROBABILITY
Our interest in The Inflammatory Reflex is driven by experiment evidence showing that it dramatically increases Survival Probability in the face of certain lethal inflammatory challenges.
Images from two studies are shown in the image below. In both studies, survival probability was profoundly higher when The Inflammatory Reflex Motor Arm (aka, the Cholinergic Anti-Inflammatory Pathway) was triggered.
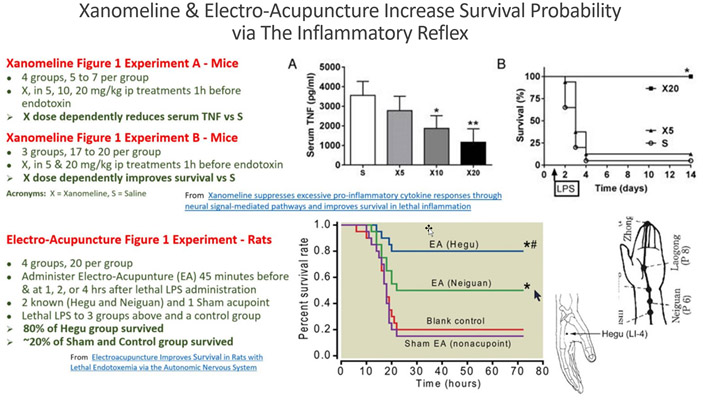
In the study on top, a drug that triggers the Muscarinic-Acetylcholine receptors in the brain, Xanomeline, was administered to mice in 2 doses along with a control group getting saline. Soon after, a lethal dose of LPS was administered to all three groups. No mice died who got the highest Xanomeline dose.
In the study at the bottom, the Hegu and Neguan acupoints were stimulated with Electro-Acupuncture. The Hegu acupoint was shown to trigger the IR Motor Arm in a way comparable to Xanomeline in the brain. Lethal LPS was administered to the 2 acupoint groups and the 2 control groups. The survival probability results are comparable to the remarkable results demonstrated in the Xanomeline experiment.
The IR mechanism is the most potent longevity promoting mechanism that most longevity science enthusiasts know nothing about.
WHAT IS THE INFLAMMATORY REFLEX?
From The inflammatory reflex (2002) by Kevin J. Tracey, one of the first papers on the topic: “Inflammation is a local, protective response to microbial invasion or injury. It must be fine-tuned and regulated precisely, because deficiencies or excesses of the inflammatory response cause morbidity and shorten lifespan. The discovery that cholinergic neurons inhibit acute inflammation has qualitatively expanded our understanding of how the nervous system modulates immune responses. The nervous system reflexively regulates the inflammatory response in real time, just as it controls heart rate and other vital functions. The opportunity now exists to apply this insight to the treatment of inflammation through selective and reversible ‘hard-wired’ neural systems.”
From the 2005 publication Autonomic neural regulation of immunity, co-authored by Kevin J. Tracey: “The inflammatory reflex is a physiological pathway in which the autonomic nervous system detects the presence of inflammatory stimuli and modulates cytokine production. Afferent signals to the brain are transmitted via the Vagus nerve, which activates a reflex response that culminates in efferent Vagus nerve signaling. Termed the ‘cholinergic anti-inflammatory pathway’, efferent activity in the Vagus nerve releases acetylcholine (ACh) in the vicinity of macrophages within the reticuloendothelial system. ACh can interact specifically with macrophage alpha7 subunits of nicotinic ACh receptors, leading to cellular deactivation and inhibition of cytokine release.
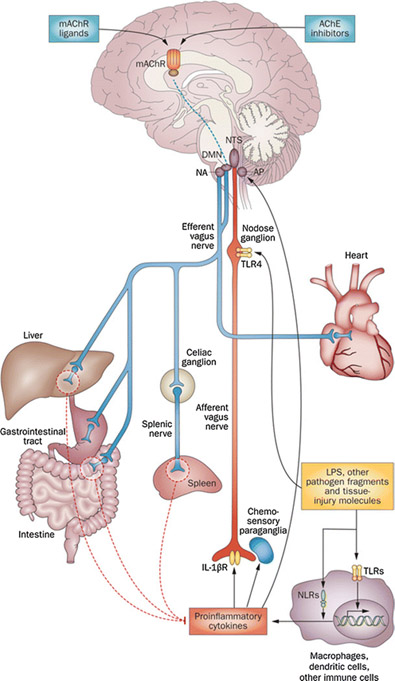
Illustration legend: Operation of the Inflammatory reflex: “The functional anatomy of the inflammatory reflex: Inflammatory mediators, such as cytokines, are released by activated macrophages and other immune cells when TLRs and NLRs are activated upon immune challenge. These mediators are detected by sensory components of the afferent arm of the inflammatory reflex (red). Neuronal interconnections between the NTS, AP, DMN, NA and higher forebrain regions (not shown) integrate afferent signaling and efferent vagus nerve-mediated immunoregulatory output. Efferent vagus nerve cholinergic output to the spleen, liver and gastrointestinal tract (blue) regulates immune activation and suppresses pro-inflammatory cytokine release (dotted red lines). Efferent vagus nerve fibers interact with the splenic nerve to suppress pro-inflammatory cytokine release in spleen—a major organ source of TNF and other pro-inflammatory cytokines in endotoxemia and other inflammatory conditions. This efferent cholinergic arm of the inflammatory reflex is termed the cholinergic anti-inflammatory pathway and can be activated in the brain through mAChR-mediated mechanisms triggered by M1 mAChR agonists and other mAChR ligands, and AChE inhibitors, such as galantamine. Abbreviations: AChE, acetylcholinesterase; AP, area postrema; DMN, dorsal motor nucleus of the vagus nerve; LPS, lipopolysaccharide (endotoxin); mAChR, muscarinic acetylcholine receptor; NA, nucleus ambiguus; NLRs, nucleotide-binding oligomerization domain-like receptors; NTS, nucleus tractus solitarius; TLR4, Toll-like receptor 4. (Adapted from [6] )”
From another early (2005) publication on the topic The cholinergic anti-inflammatory pathway. “The regulation of the innate immune response is critical for controlling inflammation and for the prevention and treatment of diseases. We recently demonstrated that the efferent vagus nerve inhibits pro-inflammatory cytokine release and protects against systemic inflammation, and termed this vagal function “the cholinergic anti-inflammatory pathway.” The discovery that the innate immune response is regulated partially through this neural pathway provides a new understanding of the mechanisms that control inflammation. In this review, we outline the cholinergic anti-inflammatory pathway and summarize the current insights into the mechanisms of cholinergic modulation of inflammation. We also discuss possible clinical implications of vagus nerve stimulation and cholinergic modalities in the treatment of inflammatory diseases.”
BACKGROUND ON THE VAGUS NERVE ANDTHE PARASYMPATHETIC AND SYMPATHETIC NERVOUS SYSTEMS
Although the vagus nerve is regarded to be part of the autonomic nervous systems, many of its actions interface with activities that are to some extent also under conscious brain control. It regulates breathing, for example, and we can to some extent override the autonomous operation to control our breathing. From Everything you need to know about the vagus nerve: “The vagus nerve is the longest and most complex of the 12 pairs of cranial nerves that emanate from the brain. It transmits information to or from the surface of the brain to tissues and organs elsewhere in the body. — The name “vagus” comes from the Latin term for “wandering.” This is because the vagus nerve wanders from the brain into organs in the neck, chest, and abdomen. It is also known as the 10th cranial nerve or cranial nerve X. — The vagus nerve has two bunches of sensory nerve cell bodies, and it connects the brainstem to the body. It allows the brain to monitor and receive information about several of the body’s different functions. — There are multiple nervous system functions provided by the vagus nerve and its related parts. The vagus nerve functions contribute to the autonomic nervous system, which consists of the parasympathetic and sympathetic parts. – The nerve is responsible for certain sensory activities and motor information for movement within the body. Essentially, it is part of a circuit that links the neck, heart, lungs, and the abdomen to the brain. The vagus nerve has a number of different functions. The four key functions of the vagus nerve are:
- Sensory: From the throat, heart, lungs, and abdomen.
- Special sensory: Provides taste sensation behind the tongue.
- Motor: Provides movement functions for the muscles in the neck responsible for swallowing and speech.
- Parasympathetic: Responsible for the digestive tract, respiration, and heart rate functioning.
Its functions can be broken down even further into seven categories. One of these is balancing the nervous system. The nervous system can be divided into two areas: sympathetic and parasympathetic.
- The sympathetic side increases alertness, energy, blood pressure, heart rate, and breathing rate.
- The parasympathetic side, which the vagus nerve is heavily involved in, decreases alertness, blood pressure, and heart rate, and helps with calmness, relaxation, and digestion. As a result, the vagus nerve also helps with defecation, urination, and sexual arousal.”
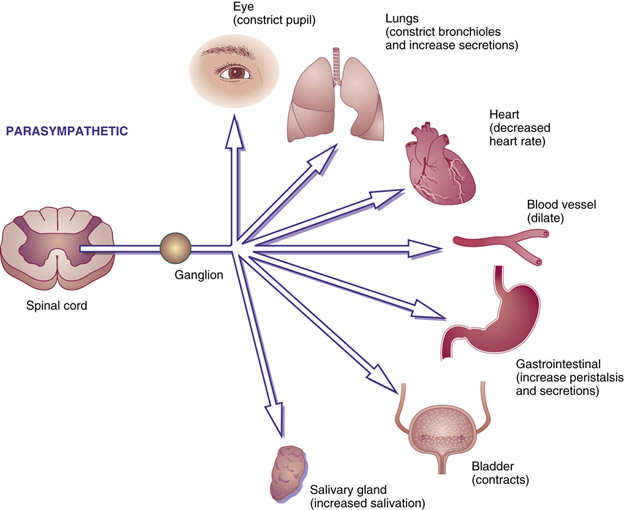 Image and legend source: Cholinergic Agonists and Antagonists – “FIG. 16.1 Parasympathetic responses. Stimulation of the parasympathetic nervous system or use of parasympathomimetic drugs will cause the pupils to constrict, bronchioles to constrict and bronchial secretions to increase, heart rate to decrease, blood vessels to dilate, peristalsis and gastric secretions to increase, the bladder muscle to contract, and salivary glands to increase salivation.”
Image and legend source: Cholinergic Agonists and Antagonists – “FIG. 16.1 Parasympathetic responses. Stimulation of the parasympathetic nervous system or use of parasympathomimetic drugs will cause the pupils to constrict, bronchioles to constrict and bronchial secretions to increase, heart rate to decrease, blood vessels to dilate, peristalsis and gastric secretions to increase, the bladder muscle to contract, and salivary glands to increase salivation.”
Key Vagus nerve functions include:
- Memory formation and consolidation, including via release of the neurotransmitter norepinephrine into the amygdala, which consolidated memories.
- Communication between the brain and the key organs: g. The vagus nerve delivers information from the gut to the brain. “Your gut uses the vagus nerve like a walkie-talkie to tell your brain how you’re feeling via electric impulses called “action potentials.” Your gut feelings are very real(ref).”
- Managing heart rate and blood pressure: The vagus nerve controls heart pumping action via electrical impulses to specialized muscle tissue. The heart does not work like a metronome and regular timing of heart beat is bad. Exact time of triggering each individual heart beat is calculated based on instantaneous values of inputs to the vagus system from multiple organs. Difference in such timing is known as heart rate variability, HRV, which is a measure of the effectiveness of vagus nerve operation and an important measure of general health and predicted longevity. HRV is a measurement of the reseliance of one’s heart and vagus nerve. High HRV is predictive of good health and longevity, while consistently low HRV is predictive of high mortality.
[[Personal note: I (Vince) wear an Oura ring which samples and monitors my HRV along with heart rate and other bioindicators 24-7. Every morning I check what the overnight HRV pattern has been and the average overnight HRV score. This represents the average variation of heart beat timing for R to R intervals measured in milliseconds. My overnight average varies up over 150 to on a very good day down to less than 70 on a so-so day, and averages out to 119. It was 136 for last night. Given that the average HRV value for males my age is under 54, I think I am doing very well in this regard. The Oura software on my cell phone computes a daily measure of my readiness to meet the coming day’s challenges, in significant part based on my recent HRV pattern. My readiness score this morning was 90, which is regarded to be optimal.
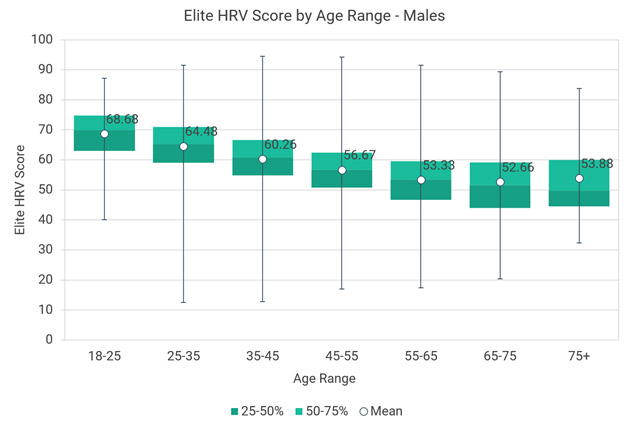
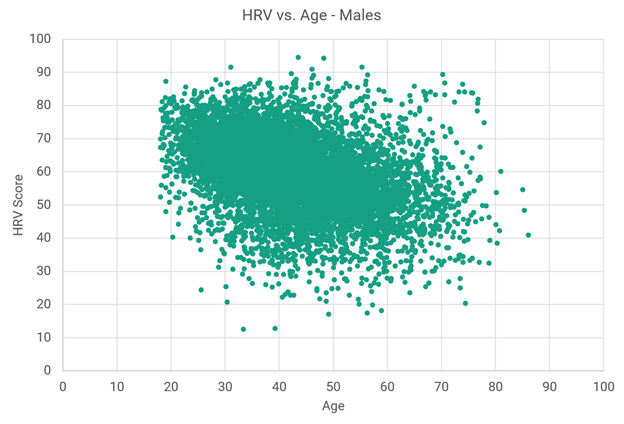
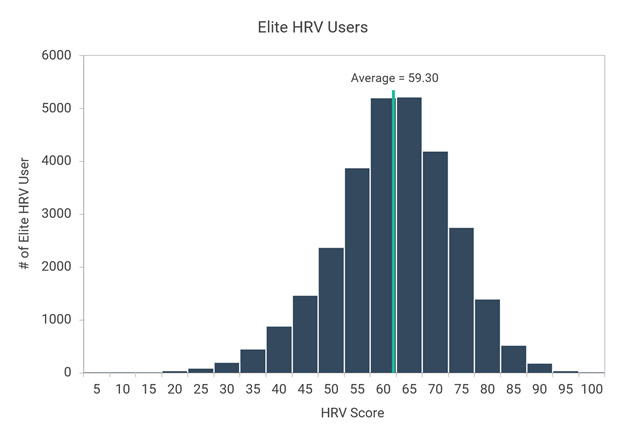
Images source Data for Elite HRV users. “Chart 2 – This chart shows Elite HRV scores statistics for 8,873 males over different age ranges. The data is presented as box plots that represent the medians, 1st and 3rd quartiles, and extreme values (minimums and maximums). There are also markers for means at each age range.” However note from the following scatter-graph that he Elite data does not include anybody as old as me, now close to 92. My average score of 119 would be high off the chart.
You might also note that nobody in my age range of 92 was sampled in this chart of Elite users and that for some reason my personal score is high off their charts. Some of the difference between these scores and mine could possibly be explained by differences between Elite and Oura HRV measurement approaches.]]
Health-inducing and stress-lowering functions of the vagus system include
- Relaxation through deep breathing: The vagus nerve communicates with the diaphragm. With deep breaths, a person can feel more relaxed.
- Decreasing systemic inflammation: The vagus nerve sends anti-inflammatory signal to other parts of the body. (The Inflammatory Reflex)
- Anxiety and fear management: The vagus nerve sends information from the gut to the brain, which is linked to dealing with stress, anxiety, and fear – hence the saying, “gut feeling.” These signals help a person to recover from stressful and scary situations.” They also let you know when you are hungary.
- Initiating relaxation after stress: “When your ever-vigilant sympathetic nervous system revs up the fight or flight responses—pouring the stress hormone cortisol and adrenaline into your body—the vagus nerve tells your body to chill out by releasing acetylcholine. The vagus nerve’s tendrils extend to many organs, acting like fiber-optic cables that send instructions to release enzymes and proteins like prolactin, vasopressin, and oxytocin, which calm you down. People with a stronger vagus response may be more likely to recover more quickly after stress, injury, or illness(ref).”
“Overstimulation of the vagus nerve is the most common cause of fainting. If you tremble or get queasy at the sight of blood or while getting a flu shot, you’re not weak. You’re experiencing “vagal syncope.” Your body, responding to stress, overstimulates the vagus nerve, causing your blood pressure and heart rate to drop. During extreme syncope, blood flow is restricted to your brain, and you lose consciousness. But most of the time you just have to sit or lie down for the symptoms to subside(ref).”
Electrical stimulation of the vagus nerve can reduce unwanted inflammation and might stop it altogether, according to an emerging body of experimental evidence. See this list of recent publications related to therapeutic electric stimulation of the vagus nerve. As a matter of fact “A burgeoning field of medical study, known as bioelectronics, may be the future of medicine. using implants that deliver electric impulses to various body parts, including the vagus nerve, scientists and doctors hope to treat illness with fewer medications and fewer side effects(ref).”
M1 AND M2 MACROPHAGES AND CONTROL OF INFLAMMATION
Source of the following quote and image is the 2018 publication Phytochemicals as modulators of M1-M2 macrophages in inflammation. “Macrophages are critical mediators of the innate immune response against foreign pathogens, including bacteria, physical stress, and injury. Therefore, these cells play a key role in the “inflammatory pathway” which in turn can lead to an array of diseases and disorders such as autoimmune neuropathies and myocarditis, inflammatory bowel disease, atherosclerosis, sepsis, arthritis, diabetes, and angiogenesis. Recently, more studies have focused on the macrophages inflammatory diseases since the discovery of the two subtypes of macrophages, which are differentiated on the basis of their phenotype and distinct gene expression pattern. Of these, M1 macrophages are pro-inflammatory and responsible for inflammatory signaling, while M2 are anti-inflammatory macrophages that participate in the resolution of the inflammatory process, M2 macrophages produce anti-inflammatory cytokines, thereby contributing to tissue healing. Many studies have shown the role of these two subtypes in the inflammatory pathway, and their emergence appears to decide the fate of inflammatory signaling and disease progression. As a next step in directing the pro-inflammatory response toward the anti-inflammatory type after an insult by a foreign pathogen (e. g., bacterial lipopolysaccharide), investigators have identified many natural compounds that have the potential to modulate M1 to M2 macrophages. –”
Continuing: “Macrophages form an essential component of innate immunity by inhibiting or promoting cellular proliferation and tissue repair [1]. They are highly plastic and dynamic in nature, which has been attributed to their ease in adapting alternate phenotypes in response to various external stimuli [2]. Macrophages are distinctly subdivided into the classical M1 and alternative M2 categories, which in turn correspond to the Th1–Th2 polarization of T cells respectively (Figure (Figure1).1). This process represents the extremes of the dynamic changing state of macrophage activation [2]. Pro-inflammatory M1-macrophages release cytokines that inhibit the proliferation of malignant cells [3] and counter various pathogens [4]. In contrast, M2-macrophages or tumor-associated macrophages (TAM’s) release cytokines that promote tumor growth and dissemination along with tissue repair [5]. The M1–M2 macrophage polarization process is tightly regulated by key signaling events. The classical activation of macrophages occurs following an injury or infection by agents such as microbial products or pro-inflammatory cytokines including bacterial lipopolysaccharides (LPS), interferon-γ (IFN-γ) or tumor necrosis factor-α (TNF-α) [6]. M1 macrophages are characterized by the production of pro-inflammatory cytokines, the release of interleukin (IL)-12 and IL-23, and high levels of reactive oxygen intermediates (ROIs) and nitric oxide (NO). By contrast, M2 macrophages are activated by entirely different stimuli and are observed in the healing phase without the prevalence of infection [7]. These stimuli include IL-4 and/or IL-13, immune complexes and toll-like receptor (TLR), IL-1 receptor ligands, and IL-10. They are further characterized by the secretion of anti-inflammatory cytokines such asIL-10, chemokine (C-C motif) ligands (CCL)18 and CCL22, and the upregulation of dectin-1, mannose receptor CD206 (MRC1), scavenger receptor A, scavenger receptor B-1, CD163, C-C chemokine receptor type 2 (CCR2), C–X–C motif chemokine receptor (CXCR) 1,CXCR2 and dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) [8–9]. Moreover, M2 macrophages produce ornithine and polyamines through the arginase pathway [10] while M1 macrophages generate hazardous NO or ROI. To summarize the molecular agents released by classical M1 macrophages, pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-12, Type I IFN, C–X–C motif chemokine ligand (CXCL) 1–3, CXCL-5, and CXCL8–10 form the major pool [11]. On the other hand, the alternative M2 macrophages generate an array of anti-inflammatory cytokine such as IL-10, IL-4 and very low levels of pro-inflammatory cytokines such as IL-12 among others [12]. An optimum balance between M1 and M2 macrophage is very important at the basal, as well as advanced level, of immune regulation as any imbalance in the two states would be expected to cause the dysregulation of the immune pathway. The plasticity of macrophage transition might be attributed to the complex signaling pathways associated with the two phenotypes.”
Macrophages can be converted from one form to the other depending on environmental stimuli, a process refered to as changing the polarization of the macrophage. “Macrophage polarization has profound impacts on various physiological and pathological conditions, such as angiogenesis, wound repair, inflammation, and tumorigenesis. Regardless of physiological or pathological process, a serial of signaling pathways and diverse mediators (e.g., cytokines, chemokines, transcriptional factors) are heavily implicated in macrophage polarization. Signals from the local microenvironment are modulated by various receptors on the macrophages to initiate multiple pathways of macrophage polarization(ref).”
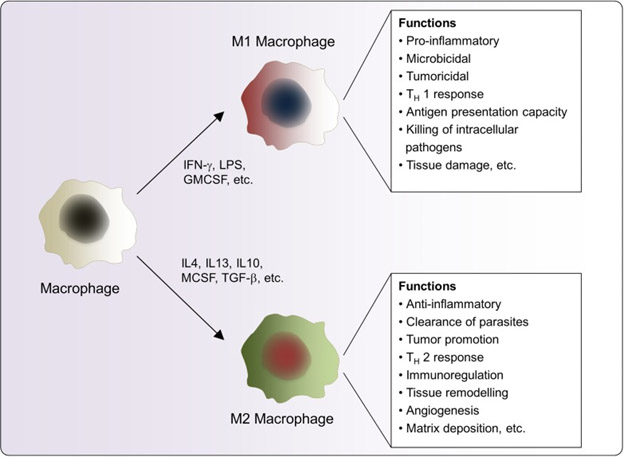
Maintaining proper balance of M1 and M2 polarization is important for health and some auto-immune diseases like lupus result in favoring M1 polarization which leads to constitutional states of inflammation. “In pathological conditions, chronic persistent inflammation could induce an aberrant response of macrophage and cause a shift in their phenotypes. Moreover, this shift would result in the alteration of macrophage polarization in some vascular dermatoses; e.g., an increase in proinflammatory M1 emerges from Behcet’s disease (BD), psoriasis, and systemic lupus erythematosus (SLE), whereas an enhancement in anti-inflammatory M2 appears in infantile hemangioma (IH).”
ROLE OF JDJM3 IN THE INFLAMMATORY REFLEX
So, for the Inflammatory Reflex operating via the cholinergic anti-inflammatory pathway to do its job on controlling inflammation, there has to be adequate M1 to M2 polarization. This is where JDJM3 comes in. Again, we recall that JDJM3 specifically relates to a specific histone position. It demethylates HK27me2-3, activating thousands of genes silenced by this methylation. There is evidence that the presence of JDJM3 is a necessary condition for adequate M1 to M2 polarization to take place. The 2010 publication The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection reports: “Polarization of macrophages to M1 or M2 cells is important for mounting responses against bacterial and helminth infections, respectively. Jumonji domain containing-3 (Jmjd3), a histone 3 Lys27 (H3K27) demethylase, has been implicated in the activation of macrophages. Here we show that Jmjd3 is essential for M2 macrophage polarization in response to helminth infection and chitin, though Jmjd3 is dispensable for M1 responses. Furthermore, Jmjd3 (also known as Kdm6b) is essential for proper bone marrow macrophage differentiation, and this function depends on demethylase activity of Jmjd3. Jmjd3 deficiency affected trimethylation of H3K27 in only a limited number of genes. Among them, we identified Irf4 as encoding a key transcription factor that controls M2 macrophage polarization. Collectively, these results show that Jmjd3-mediated H3K27 demethylation is crucial for regulating M2 macrophage development leading to anti-helminth host responses.”
While the above result may seem specific to helminth infection, the demethylation OF H3K27 and consequent activation of the Irf4 transcription factor seems to be a key factor given other pathologies as well, and we think it is fundamental for M2 macrophage polarization. For example the 2016 publication Epigenetic Modulation in Periodontitis: Interaction of Adiponectin and JMJD3-IRF4 Axis in Macrophages reports: “Emerging evidence suggests an important role for epigenetic mechanisms in modulating signals during macrophage polarization and inflammation. JMJD3, a JmjC family histone demethylase necessary for M2 polarization is also required for effective induction of multiple M1 genes by lipopolysaccharide (LPS). However, the effects of JMJD3 to inflammation in the context of obesity remains unknown. To address this deficiency, we firstly examined the expression of JMJD3 in macrophage isolated from bone marrow and adipose tissue of diet induced obesity (DIO) mice. The results indicated that JMJD3 was down-regulated in obesity. Adiponectin (APN), a factor secreted by adipose tissue which is down-regulated in obesity, functions to switch macrophage polarization from M1 to M2, thereby attenuating chronic inflammation. Intriguingly, our results indicated that APN contributed to JMJD3 up-regulation, reduced macrophage infiltration in obese adipose tissue, and abolished the up-regulation of JMJD3 in peritoneal macrophages isolated from DIO mice when challenged with Porphyromonas gingivalis LPS (pg.lps). To elucidate the interaction of APN and JMJD3 involved in macrophage transformation in the context of inflammation, we designed the loss and gain-function experiments of APN in vivo with APN(-/-) mice with experimental periodontitis and in vitro with macrophage isolated from APN(-/-) mice. For the first time, we found that APN can help to reduce periodontitis-related bone loss, modulate JMJD3 and IRF4 expression, and macrophage infiltration. Therefore, it can be inferred that APN may contribute to anti-inflammation macrophage polarization by regulating JMJD3 expression, which provides a basis for macrophage-centered epigenetic therapeutic strategies.”
There are several additional ways of promoting macrophage M2 polarization, for example by key transcription factors such as the anti-inflammatory STAT6 and the Yamanaka epigenetic cell reprogramming factor KLF4. The 2015 publication Transcription Factors STAT6 and KLF4 Implement Macrophage Polarization via the Dual Catalytic Powers of MCPIP reports: “Macrophage polarization plays a critical role in tissue homeostasis, disease pathogenesis, and inflammation and its resolution. IL-4–induced macrophage polarization involves induction of STAT6 and Krüppel-like factor 4 (KLF4), which induce each other and promote M2 polarization. However, how these transcription factors implement M2 polarization is not understood. We report that in murine macrophages MCP-1–induced protein (MCPIP), induced by KLF4, inhibits M1 polarization by inhibiting NF-κB activation and implements M2 polarization using both its deubiquitinase and RNase activities that cause sequential induction of reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, and autophagy required for M2 polarization. MCPIP also induces C/EBPβ and PPARγ, which promote M2 polarization. Macrophages from mice with myeloid-targeted overexpression of MCPIP show elevated expression of M2 markers and reduced response to LPS, whereas macrophages from mice with myeloid-specific deletion of MCPIP manifest elevated M1 polarization with enhanced phagocytic activity. Thus, both in vivo and in vitro experiments demonstrate that the transcription factors STAT6 and KLF4 implement IL-4–induced M2 polarization via the dual catalytic activities of MCPIP.”Another example of an intervention promoting M2 polarization is administration of the drug Macrophage polarization plays a critical role in tissue homeostasis, disease pathogenesis, and inflammation and its resolution. IL-4–induced macrophage polarization involves induction of STAT6 and Krüppel-like factor 4 (KLF4), which induce each other and promote M2 polarization. However, how these transcription factors implement M2 polarization is not understood. We report that in murine macrophages MCP-1–induced protein (MCPIP), induced by KLF4, inhibits M1 polarization by inhibiting NF-κB activation and implements M2 polarization using both its deubiquitinase and RNase activities that cause sequential induction of reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, and autophagy required for M2 polarization. MCPIP also induces C/EBPβ and PPARγ, which promote M2 polarization. Macrophages from mice with myeloid-targeted overexpression of MCPIP show elevated expression of M2 markers and reduced response to LPS, whereas macrophages from mice with myeloid-specific deletion of MCPIP manifest elevated M1 polarization with enhanced phagocytic activity. Thus, both in vivo and in vitro experiments demonstrate that the transcription factors STAT6 and KLF4 implement IL-4–induced M2 polarization via the dual catalytic activities of MCPIP.”
Another example of an intervention promoting M2 polarization is administration of the drug Azithromycin. The 2015 publication Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype reports: “To develop novel and effective treatments for ischemic stroke, we investigated the neuroprotective effects of the macrolide antibiotic azithromycin in a mouse model system of transient middle cerebral artery occlusion. Intraperitoneal administration of azithromycin significantly reduced blood-brain barrier damage and cerebral infiltration of myeloid cells, including neutrophils and inflammatory macrophages. These effects resulted in a dose-dependent reduction of cerebral ischemic damage, and in a remarkable amelioration of neurological deficits up to 7 days after the insult. Neuroprotection was associated with increased arginase activity in peritoneal exudate cells, which was followed by the detection of Ym1- and arginase I-immunopositive M2 macrophages in the ischemic area at 24-48 h of reperfusion. Pharmacological inhibition of peritoneal arginase activity counteracted azithromycin-induced neuroprotection, pointing to a major role for drug-induced polarization of migratory macrophages towards a protective, non-inflammatory M2 phenotype.”
PHYTOSUBSTANCE PROMOTERS OF THE M2 MACROPHAGE POLARITY
I think ingestion of certain plant-based substances (phytosubstances) is the most practical and most important class of interventions to promote the M2 macrophage phenotype. I am talking about substances much discussed previously in this blog like, resveratrol, quercetin, bacopa, and milk thistle– my old herbal anti-inflammatory friends. They especially include curcumin, ashwagandha, Boswellia, and ginger, the key ingredients in the anti-inflammatory liposomal herbal preparation I have invented and which we are now selling, 4 Herb Synergy. Hitherto I have discussed the anti-inflammatory properties from numerous viewpoints, and this blog entry adds another such viewpoint which is M2 macrophage activation.
The 2018 article Phytochemicals as modulators of M1-M2 macrophages in inflammation. “In this review, we provide a focused discussion of advances in the identification of natural therapeutic molecules with anti-inflammatory properties that modulate the phenotype of macrophages from M1 to M2.” The article concludes “On exposure to external stimuli, macrophages can differentiate into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes. However, the molecular mechanisms arising from or leading to these diversions are still only partially understood. It is a common knowledge that microbial products such as LPS or Th1 cytokines including TNF and IL-6 polarize macrophages toward the M1 type thereby releasing pro-inflammatory cytokines responsible for initiating an inflammatory cascade that clears the invading microbes. In contrast, the Th2 cytokines including IL-4 and IL-13 polarize the macrophages to the M2 type, which release anti-inflammatory cytokines, thereby contributing to tissue repair and remodeling [1–2]. Recently, many breakthrough discoveries have been made in which investigators have identified therapeutically important natural compounds and molecules that have the ability to pharmacologically modulate this interconversion, particularly towards the M2 phenotype. These natural modulators include chemical entities from various classes, including stilbenes, polyphenols, flavonoids, terpenes, anthraquinones and various others from diverse origins. Investigators around the world have independently verified their pharmacological activities for M1–M2 polarization, with clear-cut roles as anti-inflammatory agents. Apart from the natural sources, few studies have also successfully demonstrated the pharmacological activities of compounds isolated from fungi and other microbes as well as laboratory synthesized compounds and known drugs. These miscellaneous compounds also modulate the M1 to M2 phenotypic conversion by varied pathways. In summary, the contribution of natural products as anti-inflammatory agents via the modulation of M1 and M2 phenotypes is unquestionable. Although their potent role in M1–M2 phenotypic modulation is clear, there is an unmet need for a clear-cut understanding of the exact molecular mechanisms involved in this modulation. Hence, an in-depth investigation of the molecular pathways as well as the key players involved in modulating the M1–M2 phenotypes by these agents are needed. This would pave the way not only for a better understanding of the M1–M2 phenotypic changes but would also result in the discovery of novel analogs that may be more potent in inhibiting inflammation via M1–M2 modulation.”
So where does this discussion on the Inflammatory Reflex leave me (Vince) in terms of my earlier emphasis on controlling age-related systemic inflammation using targeted plant-based phytochemicals for this purpose? And where does it leave me in terms of understanding the underlying mechanisms of operation of my 4 Herb Synergy anti-inflammatory concoction? It reinforces these.
THE ROLE OF ACETYLCHOLINE IN M1-M2 POLARIZATION AND IN THE INFLAMMATORY REFLEX
To recap, numerous publications have pointed out that in the inflammatory reflex, efferent neurons carry signals that result in acetylcholine production in specialized T cells which results in M2 polarization of macrophages resulting in control of the inflammation. Detailing this process somewhat further, from the 2011 publication α7 Nicotinic Acetylcholine Receptor (α7nAChR) Expression in Bone Marrow-Derived Non-T Cells Is Required for the Inflammatory Reflex: “ — stimulation of the vagus nerve protects from excessive cytokine production and ameliorates experimental inflammatory disease. This mechanism, the inflammatory reflex, requires the α7 nicotinic acetylcholine receptor (α7nachr), a ligand-gated ion channel expressed on macrophages, lymphocytes, neurons and other cells.” – “Recent insights in protective mechanisms have revealed that neural circuits suppress release of damaging cytokines and that neural regulation of immune cell activation is an ancient mechanism dating back to nematode worms, a primitive animal with rudimentary nervous and immune systems (10–12). — A prototypical antiinflammatory neural mechanism is the inflammatory reflex (11,13–15). Action potentials arising in the brain stem are transmitted in the cholinergic vagus nerve to terminate in the celiac ganglion, the site of origin of the adrenergic splenic nerve. Signals through the splenic nerve terminate on specialized T cells that respond to norepinephrine by producing acetylcholine, the terminal neurotransmitter in the circuit. Acetylcholine interacts with cytokine producing macrophages in the red pulp and marginal zone to suppress TNF release (15). The cytokine-suppressing mechanism of the inflammatory reflex requires the α7 nicotinic acetylcholine receptor (α7nAChR), as evidenced by the observation that the inflammatory reflex is impaired in α7nAChR-deficient mice (16). Furthermore, deleting α7nAChR from isolated macrophages impairs the ability of acetylcholine to suppress TNF and other cytokines.”
The liposomal bodies in 4 Herb Synergy and most other liposomal supplements consist of acetylcholine. However in my discussions of the impacts of the 4 Herb Synergy supplement in controlling inflammation, I (Vince) have only discussed the roles of the herbal substances carried by the liposomes. I have never hitherto mentioned the additional possible anti-inflammatory role of the acetylcholine in the liposomal bodies, although this could be very significant.
We note for completeness here that synthesis of acetylcholine by macrophages can go on in other contexts besides the Inflammatory Reflex, contexts not involving the vagus nerve or neurons but relying only on chemical signaling instead. For example this 2021 article is about how cold induces certain macrophages in adipose fat to synthesize acetylcholine for purposes of getting beige fat cells to give off heat: Acetylcholine-synthesizing macrophages in subcutaneous fat are regulated by β2-adrenergic signaling. “Non-neuronal cholinergic signaling, mediated by acetylcholine, plays important roles in physiological processes including inflammation and immunity. Our group first discovered evidence of non-neuronal cholinergic circuitry in adipose tissue, whereby immune cells secrete acetylcholine to activate beige adipocytes during adaptive thermogenesis. Here, we reveal that macrophages are the cellular protagonists responsible for secreting acetylcholine to regulate thermogenic activation in subcutaneous fat, and we term these cells cholinergic adipose macrophages (ChAMs). An adaptive increase in ChAM abundance is evident following acute cold exposure, and macrophage-specific deletion of choline acetyltransferase (ChAT), the enzyme for acetylcholine biosynthesis, impairs the cold-induced thermogenic capacity of mice. Further, using pharmacological and genetic approaches, we show that ChAMs are regulated via adrenergic signaling, specifically through the β2 adrenergic receptor. These findings demonstrate that macrophages are an essential adipose tissue source of acetylcholine for the regulation of adaptive thermogenesis, and may be useful for therapeutic targeting in metabolic diseases.
The process is illustrated by this image and legend from that publication:
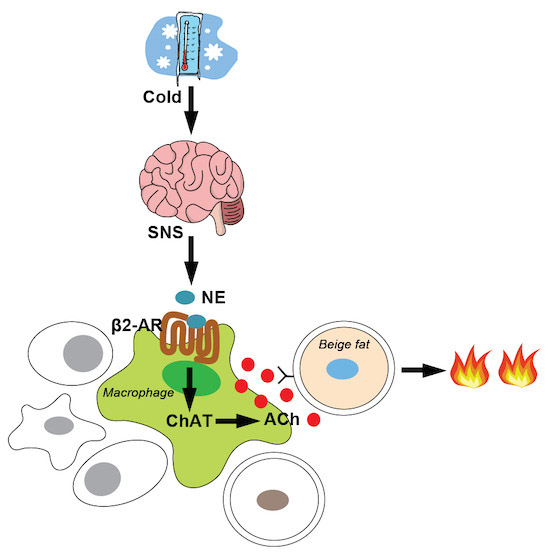
“Immune cells secrete acetylcholine to activate beige adipocytes during adaptive thermogenesis, yet the identity of these acetylcholine-secreting cells is unclear. This study shows that a subpopulation of adipose macrophages—named cholinergic adipose macrophages (ChAMs)—is responsible for β2 adrenergic receptor-dependent secretion of acetylcholine upon acute cold to stimulate thermogenesis in neighboring beige adipocytes within murine subcutaneous fat.”
We intend at some point to publish a separate blog entry specifically focused on the many health-inducing roles that can be played by acetylcholine supplementation.
A QUESTION WE ASK OURSELVES
Where does it leave us in understanding the triggering and operation of the YOUNGING 01 aging-in-reverse process we have so much discussed recently? It lends even more credence to the proposition that JDJM3 is fundamental to probably all natural body and cell-level rejuvenation processes. And that activation on JDJM3 is a necessary condition for just about any form of whole-body or whole-organ YOUNGING to take place. JDJM3 activation must be one thing going on. We are continuing our research to identify a practical sufficient condition for triggering basic multi-year YOUNGING 01. That is, what in addition must be done to assuredly trigger a 10 or 15 year process of whole-body YOUNGING 01? And Why? We will be reporting on that as we go on. We are currently delving deeper into certain transcription factors as well as molecular pathways known to be involved in body renewal processes including the Yamanaka Factors themselves, the WNT, SHH, BMP, Sonic Hedgehog, TGF-beta T-box genes and SMAD3 pathways. We are also looking deeper into growth and development factors known the be present in umbilical cord blood plasma.

