By Victor
Circadian Regulation is a fascinating topic with ties to many issues involved in antiaging research, including the regulation of stem cell populations.(See The circadian molecular clock creates epidermal stem cell heterogeneity, Biological Clock Controls Activation of Skin Stem Cells). Studies have shown that 10-15% of all cellular transcripts oscillate in a circadian manner, including 50% of all nuclear receptors. That is a huge number of genes involved in virtually all complex cellular processes in all of the cells of the body. Decline of circadian function (rhythmicity and amplitude) appears to be associated with many age-related pathologies. Resetting or re-synchronizing the clocks may be responsible for many of the benefits of calorie restriction (CR). The peripheral clocks are entrained by metabolic factors, like feeding and physical activity, while the central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus is entrained by ambient conditions, such as light and to a lesser extent temperature. Clearly, in modern lifestyles these so called “zeitgebers” are often not synchronized. The central importance of synchronicity between the peripheral and central clocks is very clearly illustrated by the dramatically elevated risks of a variety of disorders in night-shift workers. These include depression, cognitive decline, obesity, diabetes, heart disease, and cancer, mostly the same disorders associated with aging. One mystery has always been, how do circadian clocks sense and respond to metabolic factors, if they are purely transcriptional feedback oscillators?
Epigenetics and Circadian Regulation
It has been discovered that circadian oscillations are not just a matter of transcriptional feedback loops, but that epigenetic mechanisms play a crucial role. Recent research has begun to elucidate the epigenetic pathways involved in circadian regulation, such as the link between metabolism and circadian clocks.(ref, ref) Actually, a decade ago it was discovered that the central clock in the SCN is activated by an epigenetic mechanism. A single pulse of light to the optic nerve was shown to induce phosphorylation of serine 10 in H3, which directly results in circadian transcriptional activity in the hypothalamus. It also results in a 10-fold increase in acetylation of lysine-14 of H3 (H3K14).(ref) It turns out that one of the components of the canonical transcriptional clock, CLOCK, is also the HAT (histone acetyl transferase) responsible for acetylation of histones, as well as many important non-histone proteins.(ref) Acetylation is an important “switch”, which alters the activity of many proteins (p53 is one clear example of this.). If there is a cyclic writer (HAT), then there must also be an oscillating eraser, or histone deacetylase (HDAC). The natural question then is what is the corresponding eraser, or HDAC, to CLOCK? The surprising answer to this question also reveals the link between the metabolic state of a cell and circadian oscillations, elucidating how peripheral clocks can be regulated by metabolic factors.
The Missing Link
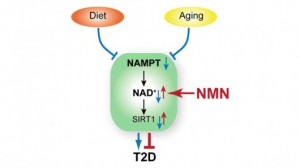
The mysterious link between metabolism and circadian regulation turns out to be the HDAC, sirt1.(ref,ref) One very surprising thing about this is that sirt1 does not oscillate in a circadian manner. However, sirtuins depend upon the essential coenzyme NAD (nicotinamide dinucleotide), which does oscillate in a very clear circadian pattern.(ref) One of the pathways by which NAD levels are regulated is the salvage pathway, which is an enzymatic feedback loop, see: Fig 2. Sirt1 links these two feedback loops, one enzymatic and the other transcriptional, and acts as a metabolic rheostat, controlling circadian function. Why exactly do levels of NAD respond to metabolic changes?
The Master Metabolic Sensor and Regulator – AMPK
NAD is the passive product of enzymatic pathways. In the salvage pathway, illustrated in the Fig2, we see that two key enzymes are involved in the production of NAD, NAMPT, which converts nicotinamide into nicotinamide mononucleotide (NMN) and NMNAT which then converts NMN into NAD. NAMPT (which is interestingly also an adipokine known as “visfatin”) is the rate-limiting step in this process; and in fact, NAD levels correlate extremely well with NAMPT levels. Moreover, NMNAT does not oscillate as does NAMPT. AMPK (AMP-activated protein kinase), which is known as the “master metabolic sensor” and plays a central role in mediating the effects of both exercise and nutrient restriction, determines the level of NAMPT. Transgenic models have demonstrated that NAMPT does not oscillate in tissues lacking AMPK. In the end, we see that AMPK, once again, plays the primary role of sensing metabolic status and regulating cellular and organismal responses through multiple pathways. In fact, AMPK regulates circadian function by direct phosphorylation of clock components completely independent of sirt1. See: AMPK Regulates Circadian Rhythms in a Tissue- and Isoform-Specific Manner.
For more information on sirt1, see: SIRT1-dependent Regulation of Chromatin and Transcription: Linking NAD Metabolism and Signaling to the Control of Cellular Functions. I have also commented on AMPK and SIRT1 in earlier blog entries.(ref, ref)
Anti-aging Implications
Why do any of these technical details about molecular pathways matter? Although, I have focused on the role of sirt1/NAD as a link between metabolism and circadian function, sirt1 and the other sirtuins (humans have a total of 7 orthologs with distinct functions) play a key role in many physiologic processes. Decreased sirtuin activity is thought to be associated with many age-related disorders. Although some have recently questioned the direct association with increased longevity, it remains clear that sirt1 mimics some of the effects of CR, and that sirt1 is necessary for many metabolic adaptations to CR. A search of posts in this blog will find many entries related to the sirtuin SIRT1, its epigenetic actions and its health and putative life-extending properties. Pharmaceutical companies have invested billions of dollars in developing sirtuin-activating compounds.
Understanding the pathways involved in sirtuin activity suggests another approach, identifying nutriceutical compounds that naturally increase sirtuin bioactivity. All sirtuins depend absolutely upon the adequate presence of NAD for their function. However, this critical cofactor is frequently not readily available. For example, NAD depletion and reduced sirtuin activity are the direct cause of cell death during acute heart failure(ref). However, NAMPT-generated NAD can protect heart tissue from damage during stress(ref), NAD protects cells from genotoxic stress by activating the mitochondrial sirtuins, sirt3 and sirt4(ref). Although NAD can be synthesized de novo from tryptophan, the salvage pathway requiring NAMPT, previously mentioned, is much more direct, and accounts for the majority of NAD. Despite its important role in NAD production, it would not be advisable to increase NAMPT activity, because of its inflammatory effects. NAMPT is a proinflammatory adipokine, which increases the activity of other proinflammatory mediators, including COX enzymes, TNFalpha.(ref) Unfortunately, direct NAD administration is also not a viable option, since doing so has been shown to cause rapid glycogen breakdown and severe hyperglycemia. An ideal solution would be to find a way to increase activation of sirtuins by promoting natural NAD biosynthesis, without increasing NAMPT activity.(ref)
Bypassing NAMPT
Although NAMPT has many other functions, its role in the maintenance of NAD levels is crucial for life and health. There are two forms of NAMPT, intracellular (iNAMPT) and extracellular (eNAMPT). Both forms are involved in NAD production. Certain tissues are more susceptible to the harmful effects of NAD depletion than others. These include cardiac tissue due to its high energy demands, as well as other tissues, which do not produce iNAMPT (in detectable quantities) such as the brain and pancreas.(ref) Such tissues are dependent upon eNAMPT for their NAD needs. One recent study has questioned whether eNAMPT increases plasma levels of NAD, which would imply an even greater vulnerability of pancreas and brain tissues to NAD shortages.(ref)
Recent genomic studies indicate that pancreatic beta cell dysfunction may be the primary cause of type-2 diabetes. It is likely that the age-related decline in systemic NAD plays an important role in the pathology of type-2 diabetes.(ref) The same is true for brain tissue. Once neurological and metabolic deterioration begin, a vicious cycle of physiological decline would ensue. As one researcher has observed: “Once pancreatic β cells and brain start having functional problems due to insufficient NAD biosynthesis, other peripheral tissues/organs would also be affected through insulin secretion and central metabolic regulation, resulting in the gradual deterioration of the physiological robustness through the entire body. Therefore, in the concept of the NAD World, aging is considered as the process in which organismal robustness gradually breaks down according to a functional hierarchy determined by the susceptibility to systemic NAD biosynthesis. Additionally, tissues that control the NAD-dependent branch of circadian clock feedback regulations throughout the body could be considered as “the aging clock”, and any imbalance in this fine-tuning system, which could be imposed by long-term nutritional and environmental perturbations, might initiate the process of organismal robustness breakdown (Fig. 4). Although further investigation will be necessary, tweaking “the aging clock”, possibly by supplementing NAD intermediates to systemic NAD biosynthesis, might be an effective way to make all of our clocks tick robustly in this otherwise inevitable process of aging.”(ref)
The suggestion is that direct supplementation of NMN, which is the rate-limiting step in NAD production, could have therapeutic potential, while effectively bypassing the need for NAMPT and its potentially harmful, proinflammatory effects. Moreover, NAMPT requires adequate supplies of ATP in order to produce NMN. This means that critical shortages NAD may result, whenever ATP becomes depleted. It is likely that NAD deficiency is a primary cause of tissue death resulting from hypoxia, following cerebral or cardiac ischemia. (Hypoxia is oxygen shortage which directly reduces the cellular production of ATP.) Another advantage of direct NMN supplementation is that by bypassing NAMPT and its need for ATP, NMN supplementation has the potential to maintain NAD levels in critical times of reduced oxygen/ATP supply.
Animal Studies of NMN Supplementation
What do we know about the effects of NMN? To-date no human trials of NMN have been conducted. However, several animal studies have shown impressive results.
In this first study, despite overexpressing sirt1, sirt1 activity decreases with age resulting in decreased GSIS (glucose-stimulated insulin secretion). NMN administration completely reversed this pathology restoring youthful pancreatic function. NMN also improved GSIS in normal (wild-type) mice. “Interestingly, plasma levels of nicotinamide mononucleotide (NMN), an important metabolite for the maintenance of normal NAD biosynthesis and GSIS in β cells, are significantly reduced in aged BESTO mice. Furthermore, NMN administration restores enhanced GSIS and improved glucose tolerance in the aged BESTO females, suggesting that Sirt1 activity decreases with advanced age due to a decline in systemic NAD biosynthesis. These findings provide insight into the age-dependent regulation of Sirt1 activity and suggest that enhancement of systemic NAD biosynthesis and Sirt1 activity in tissues such as β cells may be an effective therapeutic intervention for age-associated metabolic disorders such as type 2 diabetes.”
NMN, a Key NAD Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice
Highlights
- NAMPT-mediated NAD+ biosynthesis is compromised in metabolic organs by HFD (high fat diet)
- NMN ameliorates defects in NAD+ biosynthesis and glucose metabolism in T2D mice
- NMN enhances hepatic insulin sensitivity by reversing gene expression caused by HFD
- NMN also ameliorates defects in glucose and lipid metabolism in age-induced T2D mice
“Summary: Type 2 diabetes (T2D) has become epidemic in our modern lifestyle, likely due to calorie-rich diets overwhelming our adaptive metabolic pathways. One such pathway is mediated by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in mammalian NAD+ biosynthesis, and the NAD+-dependent protein deacetylase SIRT1. Here, we show that NAMPT-mediated NAD+ biosynthesis is severely compromised in metabolic organs by high-fat diet (HFD). Strikingly, nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD+ intermediate, ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice. NMN also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. Furthermore, NAD+ and NAMPT levels show significant decreases in multiple organs during aging, and NMN improves glucose intolerance and lipid profiles in age-induced T2D mice. These findings provide critical insights into a potential nutriceutical intervention against diet- and age-induced T2D.”
Graphical abstract is from the publication.
See also Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. “CONCLUSIONS/INTERPRETATION: Chronic fructose feeding causes severe islet dysfunction in mice. [Another of the many hazards of fructose.] Onset of beta cell failure in FRD-fed mice may occur via lowered secretion of eNAMPT, leading to increased islet inflammation and impaired beta cell function. Administration of exogenous NMN to FRD-fed mice corrects inflammation-induced islet dysfunction. Modulation of this pathway may be an attractive target for amelioration of islet dysfunction associated with inflammation.”
Wrapping it up-
Circadian clock functions are ubiquitous and impacted by aging, dietary, lifestyle and environmental conditions. They play important roles with respect to metabolism, health and disease susceptibilities. A key link between circadian regulation and metabolism appears to be the sirtuin SIRT1. Age or disease related-dysregulation of circadian metabolic control can lead to multiple kinds of havoc including type 2 diabetes. It is possible that supplementation of humans with NMN might work to prevent or serve as a therapy for diet or age-induced Type 2 diabetes. It would do that, if mouse-model results carry over to humans, by enhancing natural biosynthesis of NAD, and thereby increasing SIRT1 activity. Further, some researchers have argued that enhanced activity of SIRT1 could precipitate the same life-extending pathways that act in the case of calorie restriction or alternative day fasting. See my previous blog entries: Alternate-day Fasting – a better alternativeandMechanisms and Effects of Dietary Restriction


Pingback: The pivotal role of Nrf2. Part 1 – a new view on the control of oxidative damage and generation of hormetic effects | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Blue light, sleep, mental alertness and health | AGING SCIENCES – Anti-Aging Firewalls
Pingback: New, emerging and potential treatments for cancers: Part 1 – focus on the mTOR pathway | AGING SCIENCES – Anti-Aging Firewalls
Pingback: NAD+ an emerging framework for life health and life extension — Part 2: Deeper into the NAD World, hopeful interventions | AGING SCIENCES – Anti-Aging Firewalls
Pingback: FUNNY THINGS ARE HAPPENING TO ME ON THE WAY TO 100 - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: URL