By Vince Giuliano
This is the first post in a three-part series concerned with new, emerging and potential future treatments for cancers. This Part 1 post is concerned mainly with interventions that address the mTOR pathway, a growth pathway also of great interest from the viewpoint of longevity. This post also at least partially explains why certain familiar substances like aspirin, coffee, curcumin and green tea may convey both protection against cancers as well as a longevity benefit. The Part 2 post will be concerned with anti-cancer drug interventions that simultaneously address multiple growth pathways. There is a great deal of research literature related to both of these areas. Further, because they draw on drugs already approved for cancer treatment or for other indications, they are areas where clinical usage and experience seems to be increasing rapidly. The Part 3 blog entry will be concerned with selected less-known phytochemicals that have long been used in traditional Chinese medicine and that in recent years have been subjected to research scrutiny in China using the latest tools of Western Science. This research has revealed the biological pathways through which these plant-based substances work.
This and the Part 2 post are about hot areas of intensive research as well as practical clinical experimentation. Many of the papers cited in this blog entry were published in 2012 and a few of them were only a day old when I picked them up.
Background on mTOR
I have written blog entries discussing the mTOR pathway in a number of contexts. Of course mTOR stands for mammalian target of rapamycin and the drug rapamycin inhibits the mTOR pathway. The mTOR pathway is a growth pathway, very important in early development. However, mTOR signaling creates mischief as aging progresses, and turning off that signaling with rapamycin or other drugs can extend the healthspan and maximum lifespan of mice, by about 10%. For a general introduction I suggest readers review Longevity genes, mTOR and lifespan. Other relevant past blog entries include Viva mTOR! Caveat mTOR! and More mTOR links to aging theories .A more-recent sampler of research related to mTOR and rapamycin is offered in the blog entry The many faces of mTOR and rapamycin. Finally, the blog entry In-vivo cell reprogramming for longer lives discusses how inhibition of the mTOR pathway by the drug rapamycin increases the efficiency of cell reprogramming to full pluripotency status.
De-regulation of the mTOR signaling pathway has been found in many cancers, and inhibition of the mTOR pathway is rapidly becoming a mainline cancer treatment.
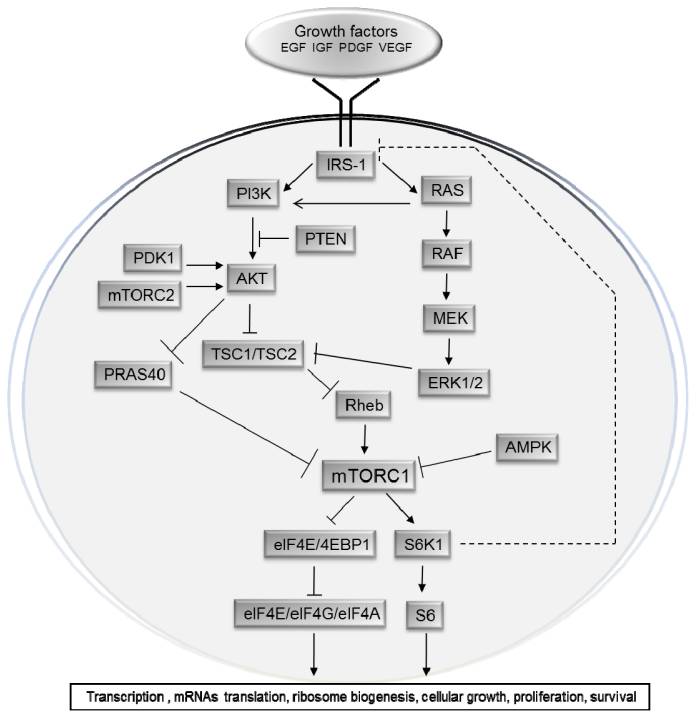
A good introduction to the topic of mTOR signaling in cancers is provided by the February 2012 publication The mTOR Signalling Pathway in Human Cancer. “The conserved serine/threonine kinase mTOR (the mammalian target of rapamycin), a downstream effector of the PI3K/AKT pathway, forms two distinct multiprotein complexes: mTORC1 and mTORC2. mTORC1 is sensitive to rapamycin, activates S6K1 and 4EBP1, which are involved in mRNA translation. It is activated by diverse stimuli, such as growth factors, nutrients, energy and stress signals, and essential signalling pathways, such as PI3K, MAPK and AMPK, in order to control cell growth, proliferation and survival. mTORC2 is considered resistant to rapamycin and is generally insensitive to nutrients and energy signals. It activates PKC-α and AKT and regulates the actin cytoskeleton. – Deregulation of multiple elements of the mTOR pathway (PI3K amplification/mutation, PTEN loss of function, AKT overexpression, and S6K1, 4EBP1 and eIF4E overexpression) has been reported in many types of cancers, particularly in melanoma, where alterations in major components of the mTOR pathway were reported to have significant effects on tumour progression. Therefore, mTOR is an appealing therapeutic target and mTOR inhibitors, including the rapamycin analogues deforolimus, everolimus and temsirolimus, are submitted to clinical trials for treating multiple cancers, alone or in combination with inhibitors of other pathways. Importantly, temsirolimus and everolimus were recently approved by the FDA for the treatment of renal cell carcinoma, PNET and giant cell astrocytoma. – Small molecules that inhibit mTOR kinase activity and dual PI3K-mTOR inhibitors are also being developed. In this review, we aim to survey relevant research, the molecular mechanisms of signalling, including upstream activation and downstream effectors, and the role of mTOR in cancer, mainly in melanoma.” – “An understanding of the mechanisms by which cells receive and integrate extracellular signals, triggering a cascade of intracellular signals that influence cell growth and metabolism, is essential to developing a well-targeted chemotherapy. One of these mechanisms is the mTOR signalling pathway, which links growth factors, nutrients and energy availability to cell survival, growth, proliferation, and motility (reviewed in refs. [1–3]).” The article also describes in detail how mTOR consists of two distinct protein complexes: mTORC1 and mTORC2 (Figure 2) [11,12], and how these differ in activation patterns and functionality.
For diagrams and discussions of how mTOR and its principal components mTORC1 and mTORC2 work, see the blog entry The many faces of mTOR and rapamycin. Another diagram focused on mTORC1 is:
Image from The mTOR Signalling Pathway in Human Cancer. “Diagram of the mTOR signalling pathway. — mTOR is a central regulator of cell growth and proliferation in response to environmental and nutritional conditions. The mTOR signalling pathway is regulated by growth factors, amino acids, and ATP and O2 levels. Signalling through mTOR modulates several downstream pathways that regulate cell-cycle progression, translation initiation, transcriptional stress responses, protein stability, and survival of cells.”
Activation of the AMPK pathway plays a key role in inhibiting mTOR signaling.
The 2008 publication AMPK phosphorylation of raptor mediates a metabolic checkpoint reports: “AMPK is a highly conserved sensor of cellular energy status that is activated under conditions of low intracellular ATP. AMPK responds to energy stress by suppressing cell growth and biosynthetic processes, in part through its inhibition of the rapamycin-sensitive mTOR (mTORC1) pathway. AMPK phosphorylation of the TSC2 tumor suppressor contributes to suppression of mTORC1; however, TSC2-deficient cells remain responsive to energy stress. Using a proteomic and bioinformatics approach, we sought to identify additional substrates of AMPK that mediate its effects on growth control. We report here that AMPK directly phosphorylates the mTOR binding partner raptor on two well-conserved serine residues, and this phosphorylation induces 14-3-3 binding to raptor. The phosphorylation of raptor by AMPK is required for the inhibition of mTORC1 and cell-cycle arrest induced by energy stress. These findings uncover a conserved effector of AMPK that mediates its role as a metabolic checkpoint coordinating cell growth with energy status.”
AMPK is discussed in my February 2012 blog entry The pivotal role of Nrf2. Part 3 – Part 3 – Is promotion of Nrf2 expression a viable strategy for human human healthspan and lifespan extension? There I pointed out how AMPK expression appears to control the aging process. “Many studies with lower organisms have revealed that increased AMPK activity can extend the lifespan. Experiments in mammals have demonstrated that AMPK controls autophagy through mTOR and ULK1 signaling which augment the quality of cellular housekeeping.” Also, this pathway is discussed in the 2010 blog entry AMPK and longevity and in Victor’s January 2012 blog entry Circadian Regulation, NMN, Preventing Diabetes, and Longevity. The AMPK pathway is activated by exercise, PGC -1alpha and by numerous phytosubstances.
Cancer cells are resistant to the effects of DNA-damaging drugs because elevated levels of mTOR in cancers inhibit SIRT1 allowing senescent P53-deficient cancer cells to evade apoptosis and re-enter the cell cycle. Treatment with rapamycin or other mTOR inhibitors averts this resistance.
The 2011 publication Cancer cell survival following DNA damage-mediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent Inhibition of sirtuin 1 reports:.”DNA-damaging agents can induce premature senescence in cancer cells, which contributes to the static effects of cancer. However, senescent cancer cells may re-enter the cell cycle and lead to tumor relapse. Understanding the mechanisms that control the viability of senescent cells may be helpful in eliminating these cells before they can regrow. Treating human squamous cell carcinoma (SCC) cells with the anti-cancer compounds, resveratrol and doxorubicin, triggered p53-independent premature senescence by invoking oxidative stress-mediated DNA damage. This process involved the mTOR-dependent phosphorylation of SIRT1 at serine 47, resulting in the inhibition of the deacetylase activity of SIRT1. SIRT1 phosphorylation caused concomitant increases in p65/RelA NF-κB acetylation and the expression of an anti-apoptotic Bfl-1/A1. SIRT1 physically interacts with the mTOR-Raptor complex, and a single amino acid substitution in the TOS (TOR signaling) motif in the SIRT1 prevented Ser-47 phosphorylation and Bfl-1/A1 induction. The pharmacologic and genetic inhibition of mTOR, unphosphorylatable S47A, or F474A TOS mutants restored SIRT1 deacetylase activity, blocked Bfl-1/A1 induction, and sensitized prematurely senescent SCC cells for apoptosis. We further show that the treatment of UVB-induced SCCs with doxorubicin transiently stabilized tumor growth but was followed by tumor regrowth upon drug removal in p53(+/-)/SKH-1 mice. The subsequent treatment of stabilized SCCs with rapamycin decreased tumor size and induced caspase-3 activation. These results demonstrate that the inhibition of SIRT1 by mTOR fosters survival of DNA damage-induced prematurely senescent SCC cells via Bfl-1/A1 in the absence of functional p53.”
There are several approved drug substances that inhibit of mTOR signaling
Known substances that inhibit the mTOR pathway include Rapamycin itself (in drug firm known as Sirolimus), and drug analogs of Sirolimus including Temsirolimus and Everolimus.
The March 8 2012 publication Antitumor activities of ATP-competitive inhibitors of mTOR in colon cancer cellsreports: “Background: The mammalian target of rapamycin (mTOR) is frequently activated in colon cancers due to mutations in the phosphatidylinositol 3-kinase (PI3K) pathway. Targeting mTOR with allosteric inhibitors of mTOR such as rapamycin reduces colon cancer progression in several experimental models. Recently, a new class of mTOR inhibitors that act as ATP-competitive inhibitors of mTOR, has been developed. The effectiveness of these drugs in colon cancer cells has however not been fully characterized. Methods: LS174T, SW480 and DLD-1 colon cancer cell lines were treated with PP242 an ATP-competitive inhibitor of mTOR, NVP-BEZ235, a dual PI3K/mTOR inhibitor or rapamycin. Tumor cell growth, proliferation and survival were assessed by MTS assay, 5-bromo-2′ -deoxyuridine (BrDU) incorporation or by quantification of DNA fragmentation respectively. In vivo, the anticancer activity of mTOR inhibitors was evaluated on nude mice bearing colon cancer xenografts. Results: PP242 and NVP-BEZ235 reduced the growth, proliferation and survival of LS174T and DLD-1 colon cancer cells more efficiently than rapamycin. Similarly, PP242 and NVP-BEZ235 also decreased significantly the proliferation and survival of SW480 cells which were resistant to the effects of rapamycin. In vivo, PP242 and NVP-BEZ235 reduced the growth of xenografts generated from LS174T and SW480 cells. Finally, we also observed that the efficacy of ATP-competitive inhibitors of mTOR was enhanced by U0126, a MEK inhibitor. Conclusions: Taken together, these results show that ATP-competitive inhibitors of mTOR are effective in blocking colon cancer cell growth in vitro and in vivo and thus represent a therapeutic option in colon cancer either alone or in combination with MEK inhibitors.”
The February 2012 publication Aberrant activation of the mTOR pathway and anti-tumour effect of everolimus on oesophageal squamous cell carcinoma reported: “Background: The mammalian target of rapamycin (mTOR) protein is important for cellular growth and homeostasis. The presence and prognostic significance of inappropriate mTOR activation have been reported for several cancers. Mammalian target of rapamycin inhibitors, such as everolimus (RAD001), are in development and show promise as anti-cancer drugs; however, the therapeutic effect of everolimus on oesophageal squamous cell carcinoma (OSCC) remains unknown. Methods: Phosphorylation of mTOR (p-mTOR) was evaluated in 167 resected OSCC tumours and 5 OSCC cell lines. The effects of everolimus on the OSCC cell lines TE4 and TE11 in vitro and alone or in combination with cisplatin on tumour growth in vivo were evaluated. Results: Mammalian target of rapamycin phosphorylation was detected in 116 tumours (69.5%) and all the 5 OSCC cell lines. Everolimus suppressed p-mTOR downstream pathways, inhibited proliferation and invasion, and induced apoptosis in both TE4 and TE11 cells. In a mouse xenograft model established with TE4 and TE11 cells, everolimus alone or in combination with cisplatin inhibited tumour growth. Conclusion: The mTOR pathway was aberrantly activated in most OSCC tumours. Everolimus had a therapeutic effect both as a single agent and in combination with cisplatin. Everolimus could be a useful anti-cancer drug for patients with OSCC.”
The 2011 publication Effects of mTOR inhibitor everolimus (RAD001) on bladder cancer cellsreported: “Purpose: We investigated the effect of the mTOR inhibitor RAD001 (everolimus) on human bladder cancer (BC) cells in vitro and in vivo. Experimental Design: The effect of RAD001 on the growth of UM-UC-3, UM-UC-6, UM-UC-9, and UM-UC-14 BC cells were assessed by crystal violet and [(3)H]thymidine incorporation assays. Flow cytometric cell-cycle analyses were done to measure the apoptotic cell fraction. Protein synthesis was measured using tritium-labeled leucine incorporation assays. The effects of RAD001 on the mTOR pathway were analyzed by Western blotting. To test the effects of RAD001 in vivo, UM-UC-3, UM-UC-6, and UM-UC-9 cells were subcutaneously implanted into nude mice. Tumor-bearing mice were treated orally with RAD001 or placebo. Tumors were harvested for immunohistochemical analysis. Results: In vitro, RAD001 transiently inhibited BC cell growth in a dose-dependent manner. This effect was augmented by re-treatment of cells after 3 days. UM-UC-14 cells were the most sensitive to RAD001, whereas UM-UC-9 cells were the least sensitive. After re-treatment with RAD001, only sensitive cell lines showed G(1)-phase arrest, with no evidence of apoptosis. RAD001 significantly inhibited the growth of tumors that were subcutaneously implanted in mice. Inhibition of protein synthesis through the S6K and 4EBP1 pathways seems to be the main mechanism for the RAD001-induced growth inhibition. However, inhibition of angiogenesis was the predominant mechanism of the effect of RAD001 on UM-UC-9 cells. Conclusions: The mTOR inhibitor RAD001 inhibits growth of BC cells in vitro. RAD001 is effective in treating BC tumors in an in vivo nude mouse model despite the heterogeneity of in vitro responses.”
Much of the clinical use of mTOR inhibitors against cancers is together with drugs addressing other growth pathways besides mTOR. I discuss this in the following Part 2 blog entry.
Drug-induced inhibition of the mTOR channel is also useful in many non-cancer clinical situations like ones involving kidney disease or organ transplantation. . An example is discussed in the March 12 2012 publication Delayed mTOR Inhibition with Low Dose of Everolimus Reduces TGFβ Expression, Attenuates Proteinuria and Renal Damage in the Renal Mass Reduction Model .reports: “Background: The immunosuppressive mammalian target of rapamycin (mTOR) inhibitors are widely used in solid organ transplantation, but their effect on kidney disease progression is controversial. mTOR has emerged as one of the main pathways regulating cell growth, proliferation, differentiation, migration, and survival. The aim of this study was to analyze the effects of delayed inhibition of mTOR pathway with low dose of everolimus on progression of renal disease and TGFβ expression in the 5/6 nephrectomy model in Wistar rats. Methods: This study evaluated the effects of everolimus (0.3 mg/k/day) introduced 15 days after surgical procedure on renal function, proteinuria, renal histology and mechanisms of fibrosis and proliferation. Results: Everolimus treated group (EveG) showed significantly less proteinuria and albuminuria, less glomerular and tubulointerstitial damage and fibrosis, fibroblast activation cell proliferation, when compared with control group (CG), even though the EveG remained with high blood pressure. Treatment with everolimus also diminished glomerular hypertrophy. Everolimus effectively inhibited the increase of mTOR developed in 5/6 nephrectomy animals, without changes in AKT mRNA or protein abundance, but with an increase in the pAKT/AKT ratio. Associated with this inhibition, everolimus blunted the increased expression of TGFβ observed in the remnant kidney model. Conclusion: Delayed mTOR inhibition with low dose of everolimus significantly prevented progressive renal damage and protected the remnant kidney. mTOR and TGFβ mRNA reduction can partially explain this anti fibrotic effect. mTOR can be a new target to attenuate the progression of chronic kidney disease even in those nephropathies of non-immunologic origin.”
An indication of the intense current clinical interest in mTOR inhibition is the existence of 261 clinical trials.
The US government database of clinical trials lists 261 clinical trial studies related to mTOR in various stages of progress. Many of these are related to cancers including Kaposi Sarcoma, Hepatocellular Carcinoma, Endometrial Neoplasms, Prostate Cancer,Solid Tumors, Breast Cancer, Rectal Cancer, Thyroid Cancer, Renal Cancer, Kidney Cancer, Pancreatic Cancer, Cervical Cancer, Ovarian Cancer, Non-Small-Cell Lung Cancer, Neuroblastoma, Glioblastoma Multiforme; Anaplastic Astrocytoma; Anaplastic Oligodendroglioma; Malignant Glioma; Brainstem Glioma, Colorectal Cancer, Nasopharyngeal Carcinoma, Lymphomas, Acute Myelogenous Leukemia, Leiomyosarcoma; Liposarcoma; Osteosarcoma, Soft Tissue Sarcoma; Verrucous Carcinoma of the Larynx, Neuroendocrine Tumors, Recurrent Verrucous Carcinoma of the Oral Cavity, Multiple Myeloma, Mouth Neoplasms; Gastrointestinal Stromal Tumors, Head and Neck Neoplasms; Tongue Neoplasms, Squamous Cell Carcinoma, Glioblastoma Multiforme, and a number of exotic cancers such as Cowden’s Disease; and Peutz-Jeghers Syndrome. You name it. If it is a cancer, the pharma industry is betting that mTOR inhibition might help.
A commonplace drug that blocks the mTOR pathway and could be useful for inhibiting or treating cancers is Metformin, a widely-used antidiabetic drug.
The 2011 publicationMetformin: its emerging role in oncology reports: “Metformin is considered, in conjunction with lifestyle modification, as a first-line treatment modality for type 2 diabetes mellitus (DM). Recently, several clinical studies have reported reduced incidence of neoplastic diseases in DM type 2 patients treated with metformin, as compared to diet or other antidiabetic agents. Moreover, in vitro studies have disclosed significant antiproliferative and proapoptotic effects of metformin on different types of cancer. Metformin acts by activating AMP-activated protein kinase (AMPK), a key player in the regulation of energy homeostasis. Moreover, by activating AMPK, metformin inhibits the mammalian target of rapamycin complex 1 (mTORC1) resulting in decreased cancer cell proliferation. Concomitantly, metformin induces activation of LKB1 (serine/threonine kinase 11), a tumor suppressor gene, which is required for the phosphorylation and activation of AMPK. These new encouraging experimental data supporting the anti-cancer effects of metformin urgently require further clinical studies in order to establish its use as a synergistic therapy targeting the AMPK/mTOR signaling pathway.”
The March 2012 publication Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy reports: “Adenosine monophosphate-activated protein kinase (AMPK) acts as a major sensor of cellular energy status in cancers and is critically involved in cell sensitivity to anticancer agents. Here, we showed that AMPK was inactivated in lymphoma and related to the upregulation of the mammalian target of rapamycin (mTOR) pathway. AMPK activator metformin potentially inhibited the growth of B- and T-lymphoma cells. Strong antitumor effect was also observed on primary lymphoma cells while sparing normal hematopoiesis ex vivo. Metformin-induced AMPK activation was associated with the inhibition of the mTOR signaling without involving AKT. Moreover, lymphoma cell response to the chemotherapeutic agent doxorubicin and mTOR inhibitor temsirolimus was significantly enhanced when co-treated with metformin. Pharmacologic and molecular knock-down of AMPK attenuated metformin-mediated lymphoma cell growth inhibition and drug sensitization. In vivo, metformin induced AMPK activation, mTOR inhibition and remarkably blocked tumor growth in murine lymphoma xenografts. Of note, metformin was equally effective when given orally. Combined treatment of oral metformin with doxorubicin or temsirolimus triggered lymphoma cell autophagy and functioned more efficiently than either agent alone. Taken together, these data provided first evidence for the growth-inhibitory and drug-sensitizing effect of metformin on lymphoma. Selectively targeting mTOR pathway through AMPK activation may thus represent a promising new strategy to improve treatment of lymphoma patients.”
In addition, a rather surprising collection of other commonplace substances can inhibit the mTOR pathway including aspirin, caffeine, and several dietary supplements including green tea, resveratrol and curcumin.
Daily low-dose aspirin may produce its significant cardiological and anti-cancer benefits through blocking mTOR signaling.
The very recent (March 2012) publication Aspirin Inhibits mTOR Signaling, Activates AMP-Activated Protein Kinase, and Induces Autophagy in Colorectal Cancer Cells reports: “Background & Aims: Aspirin reduces the incidence of and mortality from colorectal cancer (CRC) by unknown mechanisms. Cancer cells have defects in signaling via the mechanistic target of rapamycin (mTOR), which regulates proliferation. We investigated whether aspirin affects AMP-activated protein kinase (AMPK) and mTOR signaling in CRC cells. Methods: The effects of aspirin on mTOR signaling, the ribosomal protein S6, S6 kinase (S6K1), and eukaryotic translation initiation factor 4E binding protein (4E-BP)1 were examined in CRC cells by immunoblotting. Phosphorylation of AMPK was measured; the effects of loss of AMPK? on the aspirin-induced effects of mTOR were determined using small interfering (si)RNA in CRC cells and in AMPK(?1/ ?2-/-) mouse embryonic fibroblasts. LC3 and ULK1 were used as markers of autophagy. We analyzed rectal mucosa samples from patients given 600 mg aspirin, once daily for 1 week. Results: Aspirin reduced mTOR signaling in CRC cells by inhibiting the mTOR effectors S6K1 and 4E-BP1. Aspirin changed nucleotide ratios and activated AMPK in CRC cells. mTOR was still inhibited by aspirin in CRC cells following siRNA knockdown of AMPK?, indicating AMPK-dependent and AMPK-independent mechanisms of aspirin-induced inhibition of mTOR. Aspirin induced autophagy, a feature of mTOR inhibition. Aspirin and metformin (an activator of AMPK) increased inhibition of mTOR and Akt, as well as autophagy in CRC cells. Rectal mucosal samples from patients given aspirin had reduced phosphorylation of S6K1 and S6.”
Another just-today (March 21, 2012) meta study documents the anti-cancer and cardiovascular benefits of daily aspirin supplementation. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials: “Background: Daily aspirin reduces the long-term risk of death due to cancer. However, the short-term effect is less certain, especially in women, effects on cancer incidence are largely unknown, and the time course of risk and benefit in primary prevention is unclear. We studied cancer deaths in all trials of daily aspirin versus control and the time course of effects of low-dose aspirin on cancer incidence and other outcomes in trials in primary prevention. Methods: We studied individual patient data from randomised trials of daily aspirin versus no aspirin in prevention of vascular events. Death due to cancer, all non-vascular death, vascular death, and all deaths were assessed in all eligible trials. In trials of low-dose aspirin in primary prevention, we also established the time course of effects on incident cancer, major vascular events, and major extracranial bleeds, with stratification by age, sex, and smoking status. Results: Allocation to aspirin reduced cancer deaths (562 vs 664 deaths; odds ratio [OR] 0·85, 95% CI 0·76—0·96, p=0·008; 34 trials, 69 224 participants), particularly from 5 years onwards (92 vs 145; OR 0·63, 95% CI 0·49—0·82, p=0·0005), resulting in fewer non-vascular deaths overall (1021 vs 1173; OR 0·88, 95% CI 0·78—0·96, p=0·003; 51 trials, 77 549 participants). In trials in primary prevention, the reduction in non-vascular deaths accounted for 87 (91%) of 96 deaths prevented. In six trials of daily low-dose aspirin in primary prevention (35 535 participants), aspirin reduced cancer incidence from 3 years onwards (324 vs 421 cases; OR 0·76, 95% CI 0·66—0·88, p=0·0003) in women (132 vs 176; OR 0·75, 95% CI 0·59—0·94, p=0·01) and in men (192 vs 245; OR 0·77, 95% CI 0·63—0·93, p=0·008). The reduced risk of major vascular events on aspirin was initially offset by an increased risk of major bleeding, but effects on both outcomes diminished with increasing follow-up, leaving only the reduced risk of cancer (absolute reduction 3·13 [95% CI 1·44—4·82] per 1000 patients per year) from 3 years onwards. Case-fatality from major extracranial bleeds was also lower on aspirin than on control (8/203 vs 15/132; OR 0·32, 95% CI 0·12—0·83, p=0·009). Interpretation: Alongside the previously reported reduction by aspirin of the long-term risk of cancer death, the short-term reductions in cancer incidence and mortality and the decrease in risk of major extracranial bleeds with extended use, and their low case-fatality, add to the case for daily aspirin in prevention of cancer.”
This study was widely reported in the press and on TV. “After reviewing the data, the researchers found people who took aspirin daily had a 15 percent lower risk of dying from cancer, and the risk reduction climbed to 37 percent for people who took aspirin daily for 5 years or more(ref).” While benefits of aspirin supplementation has been known for some time, the evidence indicating that the benefits may be due to mTOR inhibition is quite new. There appears to be a lively debate going on weighing whether the negative side effects of taking aspirin equal or exceed the benefits.
Caffeine also inhibits the mTOR pathways and it can therefore be speculated that regular daily caffeine consumption may reduce the incidence of cancers.
The 2011 publication Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition reports: “Caffeine is one of the most frequently ingested neuroactive compounds. All known mechanisms of apoptosis induced by caffeine act through cell cycle modulation or p53 induction. It is currently unknown whether caffeine-induced apoptosis is associated with other cell death mechanisms, such as autophagy. Herein we show that caffeine increases both the levels of microtubule-associated protein 1 light chain 3-II and the number of autophagosomes, through the use of western blotting, electron microscopy and immunocytochemistry techniques. Phosphorylated p70 ribosomal protein S6 kinase (Thr389), S6 ribosomal protein (Ser235/236), 4E-BP1 (Thr37/46) and Akt (Ser473) were significantly decreased by caffeine. In contrast, ERK1/2 (Thr202/204) was increased by caffeine, suggesting an inhibition of the Akt/mTOR/p70S6K pathway and activation of the ERK1/2 pathway. Although insulin treatment phosphorylated Akt (Ser473) and led to autophagy suppression, the effect of insulin treatment was completely abolished by caffeine addition. Caffeine-induced autophagy was not completely blocked by inhibition of ERK1/2 by U0126. Caffeine induced reduction of mitochondrial membrane potentials and apoptosis in a dose-dependent manner, which was further attenuated by the inhibition of autophagy with 3-methyladenine or Atg7 siRNA knockdown. Furthermore, there was a reduced number of early apoptotic cells (annexin V positive, propidium iodide negative) among autophagy-deficient mouse embryonic fibroblasts treated with caffeine than their wild-type counterparts. These results support previous studies on the use of caffeine in the treatment of human tumors and indicate a potential new target in the regulation of apoptosis.”
Another substance that inhibits mTOR signaling is curcumin.
The second part of the blog entry Curcumin, cancer and longevity contains a well-documented discussion of how curcumin inhibits mTOR expression. That article also outlines how curcumin is effective against many cancers. Glioblastoma, a deadly brain cancer, is an example. The blog entry quotes from the 2010 publication The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. And it also points out curcumin is effective for killing Acute lymphoblastic leukemia (ALL) cells, quoting from the 2008 publication Curcumin inhibits proliferation and induces apoptosis of leukemic cells expressing wild-type or T315I-BCR-ABL and prolongs survival of mice with acute lymphoblastic leukemia. And that blog entry it discusses how curcumin is effective against breast cancer stem cells.
A large number of other health-producing phytosubstances are known to activate the AMPK pathway and therefore serve to inhibit mTOR expression. Many of these, no surprise, are also known create apoptosis in cancer cells. I discuss only examples such substances here, anthocyanins, green tea and resveratrol.
Anthocyanins
The 2010 publication Anthocyanins target AMPK/mTOR and AMPK/Wnt pathways in exerting anti-tumor effects in colon cancer or hepatocarcinoma cells reports: “AMP-activated kinase, a sensor of cellular energy status, has emerged as a potent target for cancer prevention and/or treatment. Thus, the application of dietary origin AMPK activators could link to an effective strategy of cancer control. We have found that the activation of AMPK with anthocyanin extracted from Meoru exerted growth inhibitory effects through regulation of mTOR or GSK3β/β-catenin pathway in HT-29 colon and Hep3B cells respectively. In both types of cancer cells, the growth signal IGF-1 stimulated mTOR or Wnt pathway components. AMPK appeared to inhibit phosphorylation of mTOR possibly through interacting with one of the subunit, raptor. The effect of anthocyanins on cancer cell survival and AMPK/mTOR pathway was compared with a classical mTOR inhibitor rapamycin, and anthocyanins were found to inhibit growth through mTOR comparable to rapamycin. Moreover, anthocyanins stimulated β-catenin degradation through GSK3β activation, and it seemed to be regulated by AMPK. This work has shown that the cell energy controller AMPK can control two important cell growth regulators mTOR and Wnt, and the modulation of AMPK/mTOR or AMPK/Wnt pathways by phytochemicals such as anthocyanins can further strengthen the use of phytochemicals for cancer control.”
Green Tea
The 2011 publication Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor relates: The PI3K signaling pathway is activated in a broad spectrum of human cancers, either directly by genetic mutation or indirectly via activation of receptor tyrosine kinases or inactivation of the PTEN tumor suppressor. The key nodes of this pathway have emerged as important therapeutic targets for the treatment of cancer. In this study, we show that (-)-epigallocatechin-3-gallate (EGCG), a major component of green tea, is an ATP-competitive inhibitor of both phosphoinositide-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) with K(i) values of 380 and 320nM respectively. The potency of EGCG against PI3K and mTOR is within physiologically relevant concentrations. In addition, EGCG inhibits cell proliferation and AKT phosphorylation at Ser473 in MDA-MB-231 and A549 cells. Molecular docking studies show that EGCG binds well to the PI3K kinase domain active site, agreeing with the finding that EGCG competes for ATP binding. Our results suggest another important molecular mechanism for the anticancer activities of EGCG”
Resveratrol
A number of publications document the effect of resveratrol on inhibiting the mTOR pathway. These include:
Resveratrol inhibits protein translation in hepatic cells.(Dec 2011)
AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. (Dec 2011)
Resveratrol inhibits mTOR signaling by targeting DEPTOR. (July 2011)
Resveratrol inhibits protein translation in hepatic cells. (Dec 2011)
Some personal thoughts and questions
The research described in this blog entry, much of it quite new, points out a central molecular pathway that produces health and longevity as well as protection against cancers: MAPK activation resulting in mTOR signal suppression. The research provides an additional pillar of scientific grounding for the anti-aging lifestyleand dietary supplementregimens described in my online treatise ANTI-AGING FIREWALLS – THE SCIENCE AND TECHNOLOGY OF LONGEVITY.
When I first learned about mTOR a half-dozen years ago, I thought it was a rather exotic pathway that was mainly of interest to researchers concerned with longevity. I did not guess that mTOR inhibition would so-rapidly enter medical practice as a mainline cancer treatment. But, amazingly, that is what has happened.
Knowing the life-extending capabilities of rapamycin as documented in several mouse studies and knowing that it worked by inhibiting mTOR signaling. I have often wondered if it would be worthwhile for me to start consuming rapamycin or some other mTOR inhibitor. In the course of researching this blog entry, it dawned on me that I have already been doing exactly that, and doing it in a safe manner with a vengeance – promoting MAPK activation and consequent mTOR inhibition. My lifestyle regimen includes 45 minutes minimum of mild cardio exercise. That promotes MAPK activation as does taking the supplement PQQ(ref) and ginger(ref). I consume a couple of cups of coffee(ref) and several squares of 72% chocolate daily and sometimes chug a diet coke. So I intake plenty of caffeine resulting, as documented above, in mTOR suppression. I take substantial doses daily of green tea, curcumin and multiple anthocyanins-containing supplement tablets and gobble gobs of blueberries and eat dark leafy salads. I take resveratrol supplements. All of those things act to inhibit mTOR signaling if I am to believe the research.
Yet, as usual, I am left with a number of disquieting questions, including:
- From a viewpoint of maximizing longevity and health, how effective are the mTOR-inhibiting interventions in my lifestyle and supplement regimens compared to what may be possible? 5%, 95% or where in-between? How do I find out?
- Given all the ways that mTOR signaling can be inhibited with familiar safe substances, when and how is it best to use pharmaceutical mTOR inhibitors?
- To what extent are the longevity-producing benefits of the regimen elements mentioned above due to inhibition of mTOR or due to other pathway effects like upregulating SIRT1 or SIRT3 or downregulating expression of NF-kappaB or upregulating heme-oxygenase expression or activating heat-shock proteins?
- Or, as I suspect is the case, is the last question largely meaningless because the molecular health and longevity pathways are so inter-related?
Please stand by for Part 2 and Part 3 of this series on New, emerging and potential treatments for cancers.


Pingback: Within any cellular growth process | Lung Cancer
Pingback: New, emerging and potential treatments for cancers: Part 2 – focus on anti-cancer interventions that simultaneously address multiple growth pathways | AGING SCIENCES – Anti-Aging Firewalls
Pingback: New, emerging and potential treatments for cancers: Part 3 – selected less-known phytochemicals that have long been used in traditional Chinese medicine – focus on gambogic and gambogenic acids | AGING SCIENCES – Anti-Aging Firewalls
Wow. I’ve understood that Intermittent Fasting and Caloric Restriction is showing be cancer-preventative. Do those also affect the mTOR pathway?
Hi Vince, have you seen this? Gene Therapy to induce cells to express telomerase.
http://www.sciencedaily.com/releases/2012/05/120514204050.htm
Hi Vince,
Yes, the mTOR and AMPK is a very interesting pathway regarding homeostasis and regulating energy usage. I am not sure whether it is useful to purposely downregulate mTOR to avoid cancer etc. It is a very important process involved in cellular repair and growth. I think a consistent and constant over-expression would not be healthy, but to downgrade it too much when there is no evidence of illness, such as cancer, may be as foolhardy as having it over-expressed.
In essence, mTOR and AMPK work like a seesaw – activity in one can only rise, if the other falls. AMPK is predominantly a catabolic process. Chronic over-expression of it would lead to breakdown of muscle and organ tissue.
It really is a seesaw between the two pathways. I think the best way to balance the pathways is through intermittent fasting, where one spends most of the time in the AMPK pathway, but uses the 8 hour feeding window to stimulate repair functions via mTOR – this way, there is no chronic over-expression of either pathway. Our biology is not ‘stupid enough’ to have pathways that will destroy us, unless we do things, via behaviour/diet to get them out of kilter.
Lack of exercise, too much food (especially burgers, fries, candy, fruit juice, sugar etc…ie, what most people eat), a sendentary lifestyle, will lead to over-expression of mTOR – and that would lead to trouble. Conversly, staying in AMPK continuously would make cellular/tissue repair nigh impossible and wouild probably lead to early death for the opposite reason.
The two pathways must be balanced – at least that’s my opinion.
Electhor:
Thanks for your thoughtful comment.
I think you are right that the AMPK and mTOR pathways negatively regulate each other. And you are right about a need to keep them in balance. The basic argument for upregulating AMPK and downregulating mTOR is associated with aging and with the presence of chronic inflammatory and other conditions that could increase susceptibility to cancers. Namely, it can be useful for keeping the pathways in balance. It can be argued that increasing mTOR expression with aging, probably as a consequence of life-long epigenetic changes (DNA methylation and histone acetylation events) acts as a “pro-aging pseudo program” that also increases cancer and other age-related disease susceptibilities. And, it is probably a good idea to slow down or stop this pseudo program if possible. You can see various publications on this concept by Mikhail V. Blagosklonny. For example, see the document at http://www.impactaging.com/papers/v3/n12/full/100422.html
Your idea of intermittent fasting to balance the two pathways in an interesting and possibly very good one. I suspect that other hormetic stress interventions might also work.
You stated “Our biology is not ‘stupid enough’ to have pathways that will destroy us, unless we do things, via behaviour/diet to get them out of kilter.” I agree about the incredible wisdom of biology but a) we often unwittingly do such things in behavior/diet that gets us out of kilter and b) aging itself can significantly weaken our stress response pathways, including making intermittent fasting less effective. All of which is to say that if someone wants to live and incredibly long life like I do at the age of 83, I may need to do more than just follow the usual rules of prudence. Part of what I do involves activating stress pathways, and another part is consuming dietary polyphenols that keep my stress-response mechanisms young by reversing epigenetic changes to them. For what I do, see my recent blog entry Multifactorial hormesis II – Powerpoint presentation.
I long and very thoughtful blog entry on “Slaying two dragons with one stone” deals in detail with all of the above and is now in the works. The dragons are cancer and aging. Stand by for it. Written mainly by Jim Watson with my help, it should be published in a few days from now 4/29/2013
Vince
Upon re-reading what I had written re our biology, I admit, it was poorly expressed . I still stand by my thought that down-regulating mTOR too much may not be a good idea in all circumstances. However, your point about aging and mTOR becoming a “pro-aging pseudo program” is a very valid point. I had not thought that far ahead re getting to an advanced age and the effects mTOR may have (when taking into account life-long negative epigenome alterations).
As you pointed out in the second slides in “Multifactorial hormesis II – Powerpoint presentation.” There are some scientists who see GH/IGF-1 (plus testosterone) as pro-aging, whereas there are others that see the same hormones as anti-aging. I have seen papers supporting both stances…I guess it is one of the many things where the jury “is still out” in some respects, seeing the scientific viewpoints are the antithesis of each other.
I look forward to Jim’s blog and am sure I will get much information to contemplate.
Pingback: THE HORMESIS BARS | AGING SCIENCES – Anti-Aging Firewalls