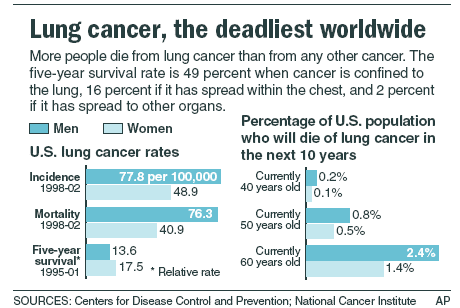
Worldwide, lung cancer is the most lethal malignancy, one responsible for more than 1.3 million deaths annually. The deaths are most-often associated with rapid spread (metastasis) of the lung cancer to multiple other body organs. Here, I review recent research on cancer stem cells in lung cancers, including important research unveiled only this week. The research offers a possible approach for prevention or cure of metastasis in lung cancers.
Basics related to lung cancers
From Wikipedia, the free encyclopedia: “Lung cancer is a disease characterized by uncontrolled cell growth in tissues of the lung. If left untreated, this growth can spread beyond the lung in a process called metastasis into nearby tissue and, eventually, into other parts of the body. Most cancers that start in lung, known as primary lung cancers, are carcinomas that derive from epithelial cells. Worldwide, lung cancer is the most common cause of cancer-related death in men and women, and is responsible for 1.3 million deaths annually, as of 2004.[1] The most common symptoms are shortness of breath, coughing (including coughing up blood), and weight loss.[2] — The main types of lung cancer are small-cell lung cancer (SCLC), also called oat cell cancer, and non-small-cell lung cancer (NSCLC). The most common cause of lung cancer is long-term exposure to tobacco smoke.[3] Nonsmokers account for 15% of lung cancer cases,[4] and these cases are often attributed to a combination of genetic factors,[5][6]radon gas,[7]asbestos,[8] and air pollution[9][10][11] including secondhand smoke.[12][13] — Lung cancer may be seen on chest radiograph and computed tomography (CT scan). The diagnosis is confirmed with a biopsy. This is usually performed by bronchoscopy or CT-guided biopsy. Treatment and prognosis depend on the histological type of cancer, the stage (degree of spread), and the patient’s general wellbeing, measured by performance status. Common treatments include surgery, chemotherapy, and radiotherapy. NSCLC is sometimes treated with surgery, whereas SCLC usually responds better to chemotherapy and radiation therapy. This is partly because SCLC often spreads quite early, and these treatments are generally better at getting to cancer cells that have spread to other parts of the body.[14] — Survival depends on stage, overall health, and other factors, but overall 14% of people in the United States diagnosed with lung cancer survive five years after the diagnosis.[2]”
On stem cells in lung cancers
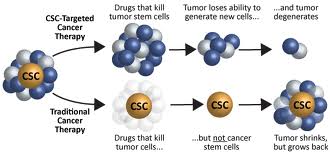
I have written several times before about CSCs (cancer stem cells). For example, their role in prostate cancer is discussed in the recent blog entry Prostate cancer – epigenetic factors, the role of Nrf2, cancer stem cells and actions of phytochemicals. See also (ref) and (ref). The most-salient considerations are a) CSCs constitute a small subpopulation of the cells in a tumor, ones with stem-cell-like properties and are capable of differentiating into active cancer cells, b) therapies that kill regular cancer cells but not CSCs may temporarily seem to clear up a cancer but relapse is highly likely as the cancer stem cells differentiate to make new cancer cells, c) CSCs are hard to kill, and d) cancer stem cells seem to play a key role in rapid metastasis of particularly malignant cancers.
On cancer stem cells in lung cancers
Although for several years the existence of lung cancer stem cells was thought by some to be controversial, the research evidence for their existence keeps piling up
Lung cancer stem cells Images source
Quoting from the March 2012 publication The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancercells: “Work in the past several years indicates that both small-cell (SCLC) and non-small cell (NSCLC) lung cancers contain stem-like cancer cells [9]–[29]. As in most other tumors, ‘lung CSCs’ have been enriched and purified using cell surface markers CD44 or CD133 or using the two functional assays mentioned above. These lung CSCs have been demonstrated to possess high clonal, clonogenic, and frequently, tumorigenic potential and to be generally resistant to therapeutic treatments. The lung cancer stem cells have been reported in long-term cultures as well as in xenografts and primary patient tumors. Of interest, a recent study using genetic mouse models of lung cancer shows that lung tumors with different genetic backgrounds have distinct CSC phenotypes [30], raising the possibility that different patient lung tumors may have different CSC phenotypes. Although the SP technique has been employed to demonstrate CSCs in several lung cancer cell lines [10], [11], [13], [25], it is not known whether all patient tumor-derived lung cancer cell lines possess a SP that is enriched in stem-like cancer cells. Here we further address this question by using the human large-cell large carcinoma line NCI-H460 (H460) and our results reveal that H460 cells possess a SP that is enriched in tumor-initiating cells.”
Also from the same publication “Lung cancer is among the most lethal malignancies with a high metastasis and recurrence rate. Recent studies indicate that tumors contain a subset of stem-like cancer cells that possess certain stem cell properties. Herein, we used Hoechst 33342 dye efflux assay and flow cytometry to isolate and characterize the side population (SP) cells from human lung cancer cell line NCI-H460 (H460). We show that the H460 SP cells harbor stem-like cells as they can readily form anchorage-independent floating spheres, possess great proliferative potential, and exhibit enhanced tumorigenicity. Importantly, the H460 SP cells were able to self-renew both in vitro and in vivo. Finally, we show that the H460 SP cells preferentially express ABCG2 as well as SMO, a critical mediator of the Hedgehog (HH) signaling, which seems to play an important role in H460 lung cancer cells as its blockage using Cyclopamine greatly inhibits cell-cycle progression. Collectively, our results lend further support to the existence of lung cancer stem cells and also implicate HH signaling in regulating large-cell lung cancer (stem) cells.”
The April 2012 publication Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446reports: “A relatively novel paradigm in tumor biology hypothesizes that cancer growth is driven by tumor cells with stem-like properties. However, direct proof of a population of stem cells in small cell lung cancer (SCLC) remains elusive. In this study, we enriched for stem-like cells from the SCLC cell line H446 by growing them as spheres in a defined serum-free medium. Sphere-derived cells have increased in vitro clonogenic and in vivo tumorigenic potentials as well as drug-resistant properties. After enrichment for stem-like cells, we used multiple candidate stem cell markers to examine the expression profile and found that the sphere-derived cells contained a higher proportion of cells expressing the stem cell surface markers uPAR and CD133 when compared with parental cells. To identify a selectable marker for the sphere-forming cells, we evaluated the sphere-forming abilities of uPAR(+) and uPAR(-) cells as well as the sphere-forming abilities of CD133(+) and CD133(-) cells. Both CD133(+) and CD133(-) cell fractions were capable of forming spheres, and no statistically significant difference was observed in the sphere-forming efficiency between these two populations. In contrast, cells derived from the uPAR(+) fraction were capable of forming spheres, whereas cells derived from the uPAR(-) fraction remained as single cells. Moreover, uPAR(+) cells efficiently formed transplantable tumors, whereas uPAR(-) cells were unable to initiate tumors when transplanted at equivalent cell numbers. In addition, uPAR(+) cells could differentiate into CD56(+) cells, CK(+) cells, and uPAR(-) cells. These data support the existence of a population of tumor sphere-forming cells with stem cell properties in the H446 SCLC cell line. Furthermore, the stem cell population may be enriched in cells expressing the uPAR cell surface marker.”
The November 2011 publication [Preneoplastic lesions of pulmonary carcinoma] reports: “The World Health Organization (WHO) 2004 classification includes 3 categories of pulmonary preneoplastic lesions, including squamous dysplasia and carcinoma in situ (CIS) for squamous cell carcinoma, atypical adenomatous hyperplasia (AAH) for the majority of adenocarcinomas and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) for carcinoids. The distinction of the 3 grades of squamous dysplasia and CIS is mainly based on the degree by which the basal cell zone is expanded, the degree of cellular atypia and the level of mitoses. The category AAH consists of a proliferation of atypical epithelial cells with Clara cells or type 2 pneumocyte features. They grow along the alveolar septae in a lepidic fashion, sometimes reaching into the terminal bronchioles. In contrast to the newly described adenocarcinoma in situ (AIS), AAH is smaller (≤ 5 mm), has a lower cell density and a lower degree of cellular atypia. The putative cancer stem cells of peripheral adenocarcinomas reside in the bronchioloalveolar duct junction, while those of central squamous cell carcinomas are located in the basal cell compartment of the bronchi. This review provides an overview of the current knowledge on preneoplastic lesions of the lungs and their clinical impact.”
Epithelial-mesenchymal transition is an important step in creating squamous lung cancer stem cells and is driven by elevated β-catenin signaling.
The March 2012 publication β-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition reports: “Human lung cancers, including squamous cell carcinoma (SCC) are a leading cause of death and, whilst evidence suggests that basal stem cells drive SCC initiation and progression, the mechanisms regulating these processes remain unknown. In this study we show that β-catenin signalling regulates basal progenitor cell fate and subsequent SCC progression. In a cohort of preinvasive SCCs we established that elevated basal cell β-catenin signalling is positively associated with increased disease severity, epithelial proliferation and reduced intercellular adhesiveness. We demonstrate that transgene-mediated β-catenin inhibition within keratin 14-expressing basal cells delayed normal airway repair while basal cell-specific β-catenin activation increased cell proliferation, directed differentiation and promoted elements of early epithelial-mesenchymal transition (EMT), including increased Snail transcription and reduced E-cadherin expression. These observations are recapitulated in normal human bronchial epithelial cells in vitro following both pharmacological β-catenin activation and E-cadherin inhibition, and mirrored our findings in preinvasive SCCs. Overall, the data show that airway basal cell β-catenin determines cell fate and its mis-expression is associated with the development of human lung cancer”
Several phyto-substances including epigallocatechin-3- gallate, curcumin, isoflavones, indole-3-carbinol, resveratrol, and isothiocyanate can limit epithelial-mesenchymal transition in cancers via modulating microRNAsin cancer cells.
The April; 19 2012 e-publication Emerging roles for modulation of microRNA signatures in cancer chemopreventionreports “miRNAs are small endogenous non-coding RNAs, approximately 21-nucleotides in length, which are shown to regulate an array of cellular processes such as differentiation, cell cycle, cell proliferation, apoptosis, and angiogenesis which are important in cancer. miRNAs can function as both tumor promoters (oncomiRs) or tumor suppressors by their ability to target numerous biomolecules that are important in carcinogenesis. Aberrant expression of miRNAs is correlated with the development and progression of tumors, and the reversal of their expression has been shown to modulate the cancer phenotype suggesting the potential of miRNAs as targets for anti-cancer drugs. Several chemopreventive phytochemicals like epigallocatechin-3- gallate, curcumin, isoflavones, indole-3-carbinol, resveratrol, and isothiocyanate have been shown to modulate the expression of numerous miRNAs in cancercells that led to either abrogation of tumor growth or sensitization of cancercells to chemotherapeutic agents. This review focuses on the putative role(s) of miRNAs in different aspects of tumorigenesis and at various stages of early drug discovery that makes them a promising class of drug targets for chemopreventive intervention in cancer. We summarize the current progress in the development of strategies for miRNA-based anti-cancer therapies. We also explore the modulation of miRNAs by various cancer chemopreventive agents and the role of miRNAs in drug metabolism. We will discuss the role of miRNAs in cancer stem cells and epithelial-to-mesenchymal transition; and talk about how modulation of miRNA expression relates to altered glycosylation patterns in cancercells. In addition, we consider the role of altered miRNA expression in carcinogenesis induced by various agents including genotoxic and epigenetic carcinogens. Finally, we will end with a discussion on the potential involvement of miRNAs in the development of cancer chemoresistance.Taken together, a better understanding of the complex role(s) of miRNAs in cancer may help in designing better strategies for biomarker discovery or drug targeting of miRNAs and/or their putative protein targets.”
MicroRNA precursor let-7 and microRNA miR-31 interact so as to determine whether lung cancer stem cells are quiescent or differentiating.
The March 2012 publication Reduced miR-31 and let-7 maintain the balance between differentiation publication Reduced and quiescence in lung cancer stem-like side population cellsreports: “Recent studies have indicated that side population (SP) cells, which are an enriched source of cancer stem cells (CSCs), drive and maintain many types of human malignancies. SP cells have distinguishing biological characteristics and are thought to contribute to metastasis, therapy resistance, and tumor recurrence. In the present study, the miRNA expression profiles of SP cells and non-SP cells were compared using miRNA array analysis. Both let-7 and miR-31 were significantly down-regulated in SP cells compared to non-SP cells. The results were confirmed by real-time PCR. Engineered repression of miR-31 caused marked repression of both lung cancer SP cell and non-SP cell growth in vitro. In contrast, engineered repression of let-7 caused marked promotion of both lung cancer SP and non-SP cells growth in vitro. Cell cycle studies further revealed that reduced miR-31 could inhibit SP cell proliferation by a cell cycle arrest in the G0/G1 phase, whereas reduced let-7 induced SP cell proliferation by accelerating G1/S phase transition. Notably, reduced miR-31 prevented SP cell differentiation, whereas reduced let-7 promoted SP cell differentiation under differentiation conditions. These findings indicate that reduced miR-31 and let-7 are involved in maintaining the balance between differentiation and quiescence in SP cells.” The suggestion is that microRNA-based therapy might be used to stem the differentiation and proliferation of lung cancer stem cells.
Conventional treatment of in non-small cell lung cancer (NSCLC) can actually foster creation of cancer stem cells.
The March 2012 publication Properties of resistant cells generated from lung cancer cell lines treated with EGFR inhibitors reports: “Background: Epidermal growth factor receptor (EGFR) signaling plays an important role in non-small cell lung cancer (NSCLC) and therapeutics targeted against EGFR have been effective in treating a subset of patients bearing somatic EFGR mutations. However, the cancer eventually progresses during treatment with EGFR inhibitors, even in the patients who respond to these drugs initially. Recent studies have identified that the acquisition of resistance in approximately 50% of cases is due to generation of a secondary mutation (T790M) in the EGFR kinase domain. In about 20% of the cases, resistance is associated with the amplification of MET kinase. In the remaining 30-40% of the cases, the mechanism underpinning the therapeutic resistance is unknown. Methods: An erlotinib resistant subline (H1650-ER1) was generated upon continuous exposure of NSCLC cell line NCI-H1650 to erlotinib. Cancer stem cell like traits including expression of stem cell markers, enhanced ability to self-renew and differentiate, and increased tumorigenicity in vitro were assessed in erlotinib resistant H1650-ER1 cells. Results: The erlotinib resistant subline contained a population of cells with properties similar to cancer stem cells. These cells were found to be less sensitive towards erlotinib treatment as measured by cell proliferation and generation of tumor spheres in the presence of erlotinib. Conclusions: Our findings suggest that in cases of NSCLC accompanied by mutant EGFR, treatment targeting inhibition of EGFR kinase activity in differentiated cancer cells may generate a population of cancer cells with stem cell properties.”
The March 2012 review publication Can lung cancerstem cells be targeted for therapies?points to the complexity of creating therapies for lung cancer based on targeting CSCs. “It is important to understand the exact role of lung CSC subpopulations in tumor initiation, recurrence, drug resistance and metastasis and explore biomarkers, signaling pathways and differentiation regulation specific to lung CSCs. Numerous measures targeting lung CSCs, e.g. genomics, proteomics and bioinformatics, have been used to investigate molecular mechanisms, eradicate cancer cells, and improve patient outcome. The present review overviewed the biological functions, biomarkers, signal pathways, differentiation regulation, genomics and proteomics, targeting roles of lung CSCs and related information on other CSCs as references. There are still a number of challenges to translate the research and understanding of lung CSCs to clinical applications and therapies, identify lung CSCs-specific and dynamic network biomarkers, study lung CSCs isolated from human samples, and clarify the source of lung CSCs. It is necessary to design effective therapies to target CSC biomarkers and signaling pathways, reverse drug resistance and induce differentiation of lung CSCs. Thus, lung CSCs as one of therapeutic target candidates for lung cancer need global forces and databases to integrate the genes, proteins, receptors, signal pathways and functions with clinical informatics and phenotypes together.”
In non-small-cell lung cancer, resistance of cancer stem cells to chemotherapy results from operation of the DNA repair machinery involving the DNA damage checkpoint protein kinase Chk1. Inhibiting Chk1 during chemotherapy can reduce survival of the cancer stem cells.
A publication pre-dated May 2012 Therapeutic targeting of Chk1 in NSCLC stem cells during chemotherapy reports: “Cancerstem cell (SC) chemoresistance may be responsible for the poor clinical outcome of non-small-cell lung cancer (NSCLC) patients. In order to identify the molecular events that contribute to NSCLC chemoresistance, we investigated the DNA damage response in SCs derived from NSCLC patients. We found that after exposure to chemotherapeutic drugs NSCLC-SCs undergo cell cycle arrest, thus allowing DNA damage repair and subsequent cell survival. Activation of the DNA damage checkpoint protein kinase (Chk) 1 was the earliest and most significant event detected in NSCLC-SCs treated with chemotherapy, independently of their p53 status. In contrast, a weak Chk1 activation was found in differentiated NSCLC cells, corresponding to an increased sensitivity to chemotherapeutic drugs as compared with their undifferentiated counterparts. The use of Chk1 inhibitors in combination with chemotherapy dramatically reduced NSCLC-SC survival in vitro by inducing premature cell cycle progression and mitotic catastrophe. Consistently, the co-administration of the Chk1 inhibitor AZD7762 and chemotherapy abrogated tumor growth in vivo, whereas chemotherapy alone was scarcely effective. Such increased efficacy in the combined use of Chk1 inhibitors and chemotherapy was associated with a significant reduction of NSCLC-SCs in mouse xenografts. Taken together, these observations support the clinical evaluation of Chk1 inhibitors in combination with chemotherapy for a more effective treatment of NSCLC.”
One approach to targeting CSCs in lung cancer in is to target the expression of telomerase in those cells.
The August 2011 publication Inhibition of telomerase activity preferentially targets aldehyde dehydrogenase-positive cancer stem-like cells in lung cancer reports: “Background: Mortality rates for advanced lung cancer have not declined for decades, even with the implementation of novel chemotherapeutic regimens or the use of tyrosine kinase inhibitors. Cancer Stem Cells (CSCs) are thought to be responsible for resistance to chemo/radiotherapy. Therefore, targeting CSCs with novel compounds may be an effective approach to reduce lung tumor growth and metastasis. We have isolated and characterized CSCs from non-small cell lung cancer (NSCLC) cell lines and measured their telomerase activity, telomere length, and sensitivity to the novel telomerase inhibitor MST312. Results: The aldehyde dehydrogenase (ALDH) positive lung cancer cell fraction is enriched in markers of stemness and endowed with stem cell properties. ALDH+ CSCs display longer telomeres than the non-CSC population. Interestingly, MST312 has a strong antiproliferative effect on lung CSCs and induces p21, p27 and apoptosis in the whole tumor population. MST312 acts through activation of the ATM/pH2AX DNA damage pathway (short-term effect) and through decrease in telomere length (long-term effect). Administration of this telomerase inhibitor (40 mg/kg) in the H460 xenograft model results in significant tumor shrinkage (70% reduction, compared to controls). Combination therapy consisting of irradiation (10Gy) plus administration of MST312 did not improve the therapeutic efficacy of the telomerase inhibitor alone. Treatment with MST312 reduces significantly the number of ALDH+ CSCs and their telomeric length in vivo. Conclusions: We conclude that antitelomeric therapy using MST312 mainly targets lung CSCs and may represent a novel approach for effective treatment of lung cancer.”
A key gene has just been discovered related to lung cancer stem cells that plays a critical role in tumor proliferation and metastasis.
I believe this is an important finding. Inhibition of expression of this gene could be the basis for a new approach to lung cancer treatment. Although the gene was first-reported in 2011, the latest publication is Matrix Metalloproteinase-10 Is Required for Lung Cancer Stem Cell Maintenance, Tumor Initiation and Metastatic Potential, dated April 24, 2012. “Matrix metalloproteinases (Mmps) stimulate tumor invasion and metastasis by degrading the extracellular matrix. Here we reveal an unexpected role for Mmp10 (stromelysin 2) in the maintenance and tumorigenicity of mouse lung cancer stem-like cells (CSC). Mmp10 is highly expressed in oncosphere cultures enriched in CSCs and RNAi-mediated knockdown of Mmp10 leads to a loss of stem cell marker gene expression and inhibition of oncosphere growth, clonal expansion, and transformed growth in vitro. Interestingly, clonal expansion of Mmp10 deficient oncospheres can be restored by addition of exogenous Mmp10 protein to the culture medium, demonstrating a direct role for Mmp10 in the proliferation of these cells. Oncospheres exhibit enhanced tumor-initiating and metastatic activity when injected orthotopically into syngeneic mice, whereas Mmp10-deficient cultures show a severe defect in tumor initiation. Conversely, oncospheres implanted into syngeneic non-transgenic or Mmp10−/− mice show no significant difference in tumor initiation, growth or metastasis, demonstrating the importance of Mmp10 produced by cancer cells rather than the tumor microenvironment in lung tumor initiation and maintenance. Analysis of gene expression data from human cancers reveals a strong positive correlation between tumor Mmp10 expression and metastatic behavior in many human tumor types. Thus, Mmp10 is required for maintenance of a highly tumorigenic, cancer-initiating, metastatic stem-like cell population in lung cancer. Our data demonstrate for the first time that Mmp10 is a critical lung cancer stem cell gene and novel therapeutic target for lung cancer stem cells.”
The news announcement in Science Daily lends texture and interpretation to this finding. “Gene Critical to Development and Spread of Lung Cancer Identified. “ScienceDaily (Apr. 24, 2012) — A single gene that promotes initial development of the most common form of lung cancer and its lethal metastases has been identified by researchers at Mayo Clinic in Florida. Their study suggests other forms of cancer may also be driven by this gene, matrix metalloproteinase-10 (MMP-10). — The study, published in the journal PLoS ONE on April 24, shows that MMP-10 is a growth factor secreted and then used by cancer stem-like cells to keep themselves vital. These cells then drive lung cancer and its spread, and are notoriously immune to conventional treatment. — The findings raise hope for a possible treatment for non-small cell lung cancer, the leading cause of U.S. cancer deaths. Researchers discovered that by shutting down MMP-10, lung cancer stem cells lose their ability to develop tumors. When the gene is given back to the cells, they can form tumors again. — The power of this gene is extraordinary, says senior investigator Alan Fields, Ph.D., the Monica Flynn Jacoby Professor of Cancer Research within the Department of Cancer Biology at Mayo Clinic in Florida. — “Our data provides evidence that MMP-10 plays a dual role in cancer. It stimulates the growth of cancer stem cells and stimulates their metastatic potential,” he says. “This helps explain an observation that has been seen in cancer stem cells from many tumor types, namely that cancer stem cells appear to be not only the cells that initiate tumors, but also the cells that give rise to metastases.” Dr. Fields says the findings were unexpected, for several reasons. The first is that the cancer stem cells express MMP-10 themselves, and use it for their own growth. Most of the known members of the matrix metalloproteinase genes are expressed in the tumor’s microenvironment, the cells and tissue that surround a tumor, he says. The enzymes produced by these genes are involved in breaking down the microenvironment that keeps a tumor in place, allowing cancer cells to spread, which is why other genes in this family have been linked to cancer metastasis. “The fact that a gene like MMP-10, which codes for a matrix metalloproteinase that has been linked to metastasis, is actually required for the growth and maintenance of cancer stem cells is very surprising. One would not have predicted that such a gene would be involved in this process,” Dr. Fields says. — The researchers also did not expect to find that cancer stem cells produce much more MMP-10 than do the rest of the cells that make up the bulk of the tumor. — “MMP-10 acts to keep these cancer stem cells healthy and self renewing, which also helps explain why these cells escape conventional chemotherapy that might destroy the rest of the tumor,” Dr. Fields says. “That is why lung cancer often recurs after treatment, and why its spread to other parts of the lung, as well as nearby lymph nodes, the brain, liver and spinal cord can’t be stopped.” — Researchers say their study suggests that MMP-10 overexpression may also be crucial to the survival of other human cancer stem cells. They observed a similar link between MMP-10 expression and the metastatic behavior and stem-like properties of human colorectal cancer, melanoma, breast, renal, and prostate cancers. — The researchers are now looking for the mechanism by which MMP-10 stimulates the growth of cancer stem cells, and are investigating the design of inhibitors that could be used to inhibit MMP-10 activity. — “Given its dual role in cancer stem cells and metastasis, targeting MMP-10 may be especially effective in treating these tumors,” Dr. Fields says.”
The important predecessor publication by the same Mayo Clinic team was the October 2011 item Matrix metalloproteinase-10 promotes Kras-mediated bronchio-alveolar stem cell expansion and lung cancer formation. “Matrix metalloproteinase 10 (MMP-10; stromelysin 2) is a member of a large family of structurally related matrix metalloproteinases, many of which have been implicated in tumor progression, invasion and metastasis. We recently identified Mmp10 as a gene that is highly induced in tumor-initiating lung bronchioalveolar stem cells (BASCs) upon activation of oncogenic Kras in a mouse model of lung adenocarcinoma. However, the potential role of Mmp10 in lung tumorigenesis has not been addressed. Here, we demonstrate that Mmp10 is overexpressed in lung tumors induced by either the smoke carcinogen urethane or oncogenic Kras. In addition, we report a significant reduction in lung tumor number and size after urethane exposure or genetic activation of oncogenic Kras in Mmp10 null (Mmp10(-/-)) mice. This inhibitory effect is reflected in a defect in the ability of Mmp10-deficient BASCs to expand and undergo transformation in response to urethane or oncogenic Kras in vivo and in vitro, demonstrating a role for Mmp10 in the tumor-initiating activity of Kras-transformed lungstem cells. To determine the potential relevance of MMP10 in human cancer we analyzed Mmp10 expression in publicly-available gene expression profiles of human cancers. Our analysis reveals that MMP10 is highly overexpressed in human lung tumors. Gene set enhancement analysis (GSEA) demonstrates that elevated MMP10 expression correlates with both cancerstemcell and tumor metastasis genomic signatures in human lungcancer. Finally, Mmp10 is elevated in many human tumor types suggesting a widespread role for Mmp10 in human malignancy. We conclude that Mmp10 plays an important role in lung tumor initiation via maintenance of a highly tumorigenic, cancer-initiating, stem-like cell population, and that Mmp10 expression is associated with stem-like, highly metastatic genotypes in human lung cancers. These results indicate that Mmp10 may represent a novel therapeutic approach to target lungcancerstem cells.”
The importance of metalloproteinases in cancers has been known for over 10 years. The 2011 publication Matrix metalloproteinases in tumorigenesis: an evolving paradigm reports: “Proteases are crucial for development, tissue remodeling, and tumorigenesis. Matrixmetalloproteinases (MMPs) family, in particular, consists of more than 20 members with unique substrates and diverse function. The expression and activity of MMPs in a variety of human cancers have been intensively studied. MMPs have well-recognized roles in the late stage of tumor progression, invasion, and metastasis. However, increasing evidence demonstrates that MMPs are involved earlier in tumorigenesis, e.g., in malignant transformation, angiogenesis, and tumor growth both at the primary and metastatic sites. Recent studies also suggest that MMPs play complex roles in tumor progression. While most MMPs promote tumor progression, some of them may protect the host against tumorigenesis in a context-dependent manner. MMPs have been chosen as promising targets for cancer therapy on the basis of their aberrant up-regulation in malignant tumors and their ability to promote cancer metastasis. Although preclinical studies testing the efficacy of MMP suppression in tumor models were so encouraging, the results of clinical trials in cancer patients have been rather disappointing. Here, we review the complex roles of MMPs and their endogenous inhibitors such as tissue inhibitors of metalloproteinase in tumorigenesis and strategies in suppressing MMPs”
MMP-9 also plays a role in the invasiveness of adenocarcinomalung cancer, and invasiveness can be inhibited via inhibition of NF-kappaB using osthole.
The April 2012 publication Osthole inhibits the invasive ability of human lung adenocarcinoma cells via suppression of NF-κB-mediated matrix metalloproteinase-9 expressionreports: “The induction of matrix metalloproteinase (MMP)-9 is particularly important for the invasiveness of various cancer cells. Osthole, a natural coumarin derivative extracted from traditional Chinese medicines, is known to inhibit the proliferation of a variety of tumor cells, but the effect of osthole on the invasiveness of tumor cells is largely unknown. This study determines whether and by what mechanism osthole inhibits invasion in CL1-5 human lung adenocarcinoma cells. Herein, we found that osthole effectively inhibited the migratory and invasive abilities of CL1-5 cells. A zymographic assay showed that osthole inhibited the proteolytic activity of MMP-9 in CL1-5 cells. Inhibition of migration, invasion, and MMP2 and/or MMP-9 proteolytic activities was also observed in other lung adenocarcinoma cell lines (H1299 and A549). We further found that osthole inhibited MMP-9 expression at the messenger RNA and protein levels. Moreover, a chromatin immunoprecipitation assay showed that osthole inhibited the transcriptional activity of MMP-9 by suppressing the DNA binding activity of nuclear factor (NF)-κB in the MMP-9 promoter. Using reporter assays with point-mutated promoter constructs further confirmed that the inhibitory effect of osthole requires an NF-κB binding site on the MMP-9 promoter. Western blot and immunofluorescence assays demonstrated that osthole inhibited NF-κB activity by inhibiting IκB-α degradation and NF-κB p65 nuclear translocation. In conclusion, we demonstrated that osthole inhibits NF-κB-mediated MMP-9 expression, resulting in suppression of lung cancer cell invasion and migration, and osthole might be a potential agent for preventing the invasion and metastasis of lung cancer.” The publication itself does not mention the role of cancer stem cells in the process but I speculate that they are involved since the MMP-9 is known to be associated with invasiveness in a number of cancer types.
In gastric and breast cancers, the compound diallyl disulfide contained in garlic is also known to control MMP-9 and cancer cell invasiveness(ref),(ref),(ref), and it appears that another garlic component S-allylcysteine can control invasiveness of non-small cell lung carcinoma. According to the 2010 publication Consumption of S-Allylcysteine Inhibits the Growth of Human Non-Small-Cell Lung Carcinoma in a Mouse Xenograft Model, “–this study investigated whether consumption of SAC (S-allylcysteine) could prevent the growth of NSCLC in both in vitro and in vivo models. It was found that SAC significantly inhibited the proliferation of human NSCLC A-549 cells in vitro. Treatment of the NF-κB inhibitor, Bay-11-7082, could significantly inhibit the proliferation of NSCLC A-549 cells. The results demonstrated that SAC significantly suppressed the activation of mTOR, NF-κB, and cyclin D1 molecules in vitro. Furthermore, the results demonstrated that consumption of SAC significantly inhibited the growth of highly metastatic human NSCLC cells in tumor-bearing mice. Bioluminescence imaging and pathological and immunohistochemical (IHC) staining results also indicated that SAC could effectively suppress the growth and malignant progression of human NSCLC in vivo. The chemopreventive effects of SAC were associated with suppression of mTOR and NF-κB molecules in vivo” Again, the publication does not mention the involvement of cancer stem cells or metalloproteinases, though I speculate that they are involved.
Curcumin and some curcumin analogs as well are known to exercise a number of anti-cancer effects including downregulation of metalloproteinase and limiting proliferation in other cancers such as in colon cancers (ref). Curcumin and other components of the spice turmeric limit the expression of MMP-3 and the invasiveness of human breast cancer, for example.(ref). “However, treatment of the cells with Cur (curcumin), DMC (demethoxycurcumin) and BDMC (bisdemethoxycurcumin ) exhibited a significant inhibition of cell invasion and motility with DMC and BDMC being more potent. These results suggest that Cur, DMC, and BDMC may be used as MMP-3 inhibitors to modulate MMP-3 expression.)”
I found an incredible number of publications on the anti-cancer and anti-invasive properties of curcumin and several other publications that link cancer stem cells to invasiveness, and others that link either curcumin or cancer cell invasiveness to metaloproteins. But I could find none none that directly links curcumin to its actions on cancer stem cells. Again, many if the dots are not connected, probably because the research on cancer stem cells is so new.
The world of lung cancer research is vast, and I have touched only on a tiny but possibly very important segment of it here. Clinicaltrials.gov lists 3967 studies for lung cancer, and 2967 studies related to cancer stem cells. 217 studies are reported related to lung cancer stem cells. So you can see how important the topic is, how highly selective I have had to be here, and how rapidly therapeutic applications related to lung cancer stem cells may be approaching the clinic. Diagnosis of metastasizing lung cancer does not have to be the sure death sentence it is now and perhaps in a few years it won’t be.
FROM TIME TO TIME, THIS BLOG DISCUSSES DISEASE PROCESSES. THE INTENTION OF THOSE DISCUSSIONS IS TO CONVEY CURRENT RESEARCH FINDINGS AND OPINIONS, NOT TO GIVE MEDICAL ADVICE. THE INFORMATION IN POSTS IN THIS BLOG IS NOT A SUBSTITUTE FOR A LICENSED PHYSICIAN’S MEDICAL ADVICE. IF ANY ADVICE, OPINIONS, OR INSTRUCTIONS HEREIN CONFLICT WITH THAT OF A TREATING LICENSED PHYSICIAN, DEFER TO THE OPINION OF THE PHYSICIAN. THIS INFORMATION IS INTENDED FOR PEOPLE IN GOOD HEALTH. IT IS THE READER’S RESPONSIBILITY TO KNOW HIS OR HER MEDICAL HISTORY AND ENSURE THAT ACTIONS OR SUPPLEMENTS HE OR SHE TAKES DO NOT CREATE AN ADVERSE REACTION