By Vince Giuliano
In past blog entries and in my treatise I have explained how I was an early adapter at taking telomerase extenders like astrogaloside4 and cycloastragenol, and why, later as a result of following much research, I stopped taking the supplements. See the discussion in my treatise under the subheading An evolving perspective on the Telomere shortening theory of aging. However, I am still beset by readers who write me wanting to know my opinion of expensive commercial supplements that are marketed specifically as telomerase activators ones like TA-65® sold by T.A. Sciences So I recently decided to visit recent research on the topic, striving to keep an open mind in the process. Here, I summarize the research cases both for taking and not taking such supplements. I have written a number of blog items on telomeres and telomerase, mostly back in 2009-2010. This blog entry provides an update. I cite a number of interesting publications that have appeared in only the last few months or weeks.
Background – Some simplified facts
For those of you unfamiliar with the topic:
- Telomeres and telomerase are relatively new, important and dynamic areas of aging science.
- Telomeres are caps at the end of chromosomes.
- Telomerase is a naturally-occurring enzyme which lengthens telomeres when activated. Germ cells and stem cells express relatively high levels of telomerase. Many normal body cells express little or no telomerase.
- Each time a cell divides the telomeres get a little shorter due to the mechanics of cell division.
- With aging after a certain number of cell divisions, telomeres in a given cell get critically short. Older people generally have shorter telomeres. Diseases, stress and a number of other conditions can also cause telomeres to shorten.
- Cells with too-short telomeres can become senescent or suffer apoptosis (die).
These simplified facts have been known for a number of years and are uncontested.
Part 1: The case for taking telomerase extender supplements
A dozen or so years ago, many researchers including the writer thought the following statements were true. It appears some researchers still subscribe to these statements although counter-arguments are presented later in this blog.
- Old-age, disease and death is possibly caused by too-short telomeres.
- Since telomere shortening is due to cell division, the telomeres get shorter and shorter with aging. This leads to dysfunctional senescent cells and tissues, to old age, to many diseases like cancer and diabetes, and to death.
- Since they are determined by cell divisions, telomere lengths are like clocks, biomarkers of aging.
- Life can therefore probably be extended and health enhanced by taking supplements specifically designed to activate telomerase and therefore keep telomeres long.
Research cited directly below here tend to support these statements. More-recent research publications such as those I review in Part 2 below suggest that these numbered statements are misleading, incorrect or both.
For the history of telomerase activators and my earlier view on the subject, see the April 2010 blog entry Telomerase activators – what do they really do? Shortly after writing that blog entry I stopped taking the activator cycloastragenol.
In some species including particular birds, there appears to be evidence that telomere lengths are roughly predictive of lifespans.
The 2010 publication Telomere dynamics rather than age predict life expectancy in the wild for Alpine Swift birds reports: “Despite accumulating evidence from in vitro studies that cellular senescence is linked to telomere dynamics, how this relates to whole-organism senescence and longevity is poorly understood and controversial. Using data on telomere length in red blood cells and long-term survival from wild Alpine swifts of a range of ages, we report that the telomere length and the rate of telomere loss are predictive of life expectancy, and that slow erosion of relatively long telomeres is associated with the highest survival probabilities. Importantly, because telomere dynamics, rather than chronological age, predict life expectancy, our study provides good evidence for a mechanistic link between telomere erosion and reduced organism longevity under natural conditions, chronological age itself possibly not becoming a significant predictor until very old ages beyond those in our sample.”
A 2010 publication Individual state and survival prospects: age, sex, and telomere length in a long-lived seabird reports for giant petrels, very long-lived seabirds: “Identifying markers that are indicative of individual state, related to fitness, and which could be used to study life-history trade-offs in wild populations is extremely difficult. Recently, it has been suggested that telomeres, the ends of eukaryote chromosomes, might be useful in this context. However, little is known of the link between telomere length and fitness in natural populations and whether it is a useful indicator of biological state. We measured average telomere length in red blood cell samples taken from a wide age range of individuals of a very long-lived and highly sexually dimorphic seabird, the southern giant petrel (Macronectes giganteus). We examined the relationship with age, sex, and subsequent survival over an 8-year period. Telomere length was longer in chicks than adults. Within the adult group, which ranged in age from 12 to 40 years, telomere length was not related to age. For the first time in birds, there was some evidence of a sex difference. Male giant petrels, which are substantially larger than females, had significantly shorter telomere lengths than females. This difference was evident from an early stage in life and is likely to relate to differences in growth trajectories. Those adults that died during the 8-year time window following the telomere length measurement had significantly shorter telomere lengths than those that survived this period, irrespective of age or sex, neither of which were significant predictors of survival. These results show that relatively short telomere length is related to future life expectancy at any adult age, demonstrating its usefulness as a state variable.”
The 2009 publicationTelomere shortening and survival in free-living corvids reports for Jackdaws: “Evidence accumulates that telomere shortening reflects lifestyle and predicts remaining lifespan, but little is known of telomere dynamics and their relation to survival under natural conditions. We present longitudinal telomere data in free-living jackdaws (Corvus monedula) and test hypotheses on telomere shortening and survival. Telomeres in erythrocytes were measured using pulsed-field gel electrophoresis. Telomere shortening rates within individuals were twice as high as the population level slope, demonstrating that individuals with short telomeres are less likely to survive. Further analysis showed that shortening rate in particular predicted survival, because telomere shortening was much accelerated during a bird’s last year in the colony. Telomere shortening was also faster early in life, even after growth was completed. It was previously shown that the lengths of the shortest telomeres best predict cellular senescence, suggesting that shorter telomeres should be better protected. We test the latter hypothesis and show that, within individuals, long telomeres shorten faster than short telomeres in adults and nestlings, a result not previously shown in vivo. Moreover, survival selection in adults was most conspicuous on relatively long telomeres. In conclusion, our longitudinal data indicate that the shortening rate of long telomeres may be a measure of ‘life stress’ and hence holds promise as a biomarker of remaining lifespan.”
Later in this blog, I cite discussions to the effect that for humans the studies on telomere lengths as predictors of lifespan yield contradictory results.
Maria Blasco has generally supported the view that extending telomeres can likely be useful in certain disease processes and possibly for retarding aging.
Maria Blasco is the leader of the Telomeres and Telomerase Group at the Spanish National Cancer Research Center. She is a highly respected and highly-published researcher who has devoted her career to telomere/telomerase science, and several of the serious research publications that are supportive of the possible positive value of taking telomerase-extending supplements have emanated from her or her group. I review some of those publications here.
There is a possibility that telomere lengths and telomerase expression can affect the ability of stem cells to regenerate tissues and thus impact on both health and aging.
This possibility was raised in the 2007 Blasco publication Telomere length, stem cells and aging: “These findings have gained special relevance as they suggest that telomerase activity and telomere length can directly affect the ability of stem cells to regenerate tissues. If this is true, stem cell dysfunction provoked by telomere shortening may be one of the mechanisms responsible for organismal aging in both humans and mice. Here, we will review the current evidence linking telomere shortening to aging and stem cell dysfunction. — The attrition of telomeric DNA that takes place during aging is likely to result from limiting amounts of telomerase activity in the adult organism, which cannot compensate for the progressive telomere shortening that occurs as cells divide during tissue regeneration4,5,23. This progressive telomere loss has been proposed to contribute to organismal aging. In turn, the vast majority of tumors and immortal cell lines have high levels of telomerase, which is thought to sustain their immortal growth by preventing telomere shortening and bypassing senescence and apoptosis23.”
In this publication, Blasco bases her findings on studies of a number of mouse models where various telomerase genes have been knocked out and on studies of progeria diseases where there is an initial deleterious mutation in one or more telomerase-related genes. For example “Figure 3 The telomerase knockout mouse as a model for telomere-induced aging. Telomere shortening in the context of Terc-deficient mice leads to premature loss of mouse viability and decreased lifespan associated with a number of degenerative pathologies. These pathologies can be rescued in the absence of p53, p21 or PMS2, which indicates that these proteins are important mediators of telomere-induced aging. Importantly, the fact that both p21 and PMS2 abrogation only rescue proliferative defects but not apoptosis triggered by short telomeres indicates that cell arrest rather than apoptosis is responsible for telomere-driven aging.”
Another publication co-authored by Blasco A p53-Dependent Response Limits Epidermal Stem Cell Functionality and Organismal Size in Mice with Short Telomeres reported: “Telomere maintenance is essential to ensure proper size and function of organs with a high turnover. In particular, a dwarf phenotype as well as phenotypes associated to premature loss of tissue regeneration, including the skin (hair loss, hair graying, decreased wound healing), are found in mice deficient for telomerase, the enzyme responsible for maintaining telomere length. Coincidental with the appearance of these phenotypes, p53 is found activated in several tissues from these mice, where is thought to trigger cellular senescence and/or apoptotic responses. Here, we show that p53 abrogation rescues both the small size phenotype and restitutes the functionality of epidermal stem cells (ESC) of telomerase-deficient mice with dysfunctional telomeres. In particular, p53 ablation restores hair growth, skin renewal and wound healing responses upon mitogenic induction, as well as rescues ESCmobilization defects in vivo and defective ESC clonogenic activity in vitro. This recovery of ESC functions is accompanied by a downregulation of senescence markers and an increased proliferation in the skin and kidney of telomerase-deficient mice with critically short telomeres without changes in apoptosis rates. Together, these findings indicate the existence of a p53-dependent senescence response acting on stem/progenitor cells with dysfunctional telomeres that is actively limiting their contribution to tissue regeneration, thereby impinging on tissue fitness.” Again, the finding applies to mice genetically deficient on telomeres. It is unclear whether it can be extended to humans with telomeres shortened by aging.”
The 2011 publication by Blasco and others A Natural Product Telomerase Activator As Part of a Health Maintenance Program is directly addresses the result of telomerase-activator supplementation in humans using TA-65®. It reports “Most human cells lack sufficient telomerase to maintain telomeres, hence these genetic elements shorten with time and stress, contributing to aging and disease. In January, 2007, a commercial health maintenance program, PattonProtocol-1, was launched that included a natural product-derived telomerase activator (TA-65®, 10–50 mg daily), a comprehensive dietary supplement pack, and physician counseling/laboratory tests at baseline and every 3–6 months thereafter. We report here analysis of the first year of data focusing on the immune system. Low nanomolar levels of TA-65® moderately activated telomerase in human keratinocytes, fibroblasts, and immune cells in culture; similar plasma levels of TA-65® were achieved in pilot human pharmacokinetic studies with single 10- to 50-mg doses. The most striking in vivo effects were declines in the percent senescent cytotoxic (CD8+/CD28−) T cells (1.5, 4.4, 8.6, and 7.5% at 3, 6, 9, and 12 months, respectively; p = not significant [N.S.], 0.018, 0.0024, 0.0062) and natural killer cells at 6 and 12 months (p = 0.028 and 0.00013, respectively). Most of these decreases were seen in cytomegalovirus (CMV) seropositive subjects. In a subset of subjects, the distribution of telomere lengths in leukocytes at baseline and 12 months was measured. Although mean telomere length did not increase, there was a significant reduction in the percent short (<4 kbp) telomeres (p = 0.037). No adverse events were attributed to PattonProtocol-1. We conclude that the protocol lengthens critically short telomeres and remodels the relative proportions of circulating leukocytes of CMV+ subjects toward the more “youthful” profile of CMV− subjects. Controlled randomized trials are planned to assess TA-65®-specific effects in humans.” Again, I point out that “Most of these decreases were seen in cytomegalovirus (CMV) seropositive subjects,” suggesting that the supplementation might be valuable for people with this pre-disease condition, but possibly less so for normally healthy people.
Another 2011 publication co-authored by Blasco and relating to TA-65® is The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. “Here, we show that a small-molecule activator of telomerase (TA-65) purified from the root of Astragalus membranaceus is capable of increasing average telomere length and decreasing the percentage of critically short telomeres and of DNA damage in haploinsufficient mouse embryonic fibroblasts (MEFs) that harbor critically short telomeres and a single copy of the telomerase RNA Terc gene (G3 Terc+/− MEFs). Importantly, TA-65 does not cause telomere elongation or rescue DNA damage in similarly treated telomerase-deficient G3 Terc−/− littermate MEFs. These results indicate that TA-65 treatment results in telomerase-dependent elongation of short telomeres and rescue of associated DNA damage, thus demonstrating that TA-65 mechanism of action is through the telomerase pathway. In addition, we demonstrate that TA-65 is capable of increasing mouse telomerase reverse transcriptase levels in some mouse tissues and elongating critically short telomeres when supplemented as part of a standard diet in mice. Finally, TA-65 dietary supplementation in female mice leads to an improvement of certain health-span indicators including glucose tolerance, osteoporosis and skin fitness, without significantly increasing global cancer incidence.”
For people who are immune system-compromised or who bear the HIV virus, supplementation with a telomerase extender could possibly be beneficial.
I wrote about this possibility over three years ago in my treatise ANTI-AGING FIREWALLS – THE SCIENCE AND TECHNOLOGY OF LONGEVITY. “One explanation for the decline in immune function with old age is cell senescence – immune cells dying or losing functional capacity because they have duplicated too many times. The same result occurs when immune system cells duplicate at a high rate to fight infections. At one time it was fashionable to talk about an immune system becoming “worn out” because of too many challenges to it due to sickness or age. Now it is more fashionable to say that the immune system T cells telomeres are too short. — Research reported by Rita Effros of UCLA and her colleagues(ref) indicates that cortisol inhibits the expression of telomerase in immune system cells, This explains why people subject to considerable stress tend to have shorter telomeres. Of course, cortisol is produced in the body in response to stress. — A therapy that enhanced expression of telomerase in immune system CD4 and CD8 cells could offer many health and longevity benefits by delaying or preventing senescence of these cells. Benefits could include less bone loss, avoidance of release of inflammatory cytokines, maintenance of strong anti-viral capability, better capability of dealing with stress, and prevention of HIV infections resulting in AIDS. A collection of studies co-authored by Rita Effros relating cell senescence to HIV pathology can be found here. — One benefit of enhancing telomerase expression in immune cells could be for patients with systemic lupus erythematosus (SLE). Many T cells divide continuously in patients with SLE, Although the natural level of expression of telomerase in CD4(+) and CD8(+) cells is high in SLE patients, it is still insufficient to prevent telomere shortening in these cells. Prevention of this shortening by telomerase activation could prevent premature senescence of these cells, and could possibly prevent some of the pathological consequences of SLE. — It is interesting that all of the major risk factors associated with cardiovascular disease (obesity, smoking, poor lipid profile, high blood pressure, diabetes and psychological stress) are associated with key markers of cellular aging (shorter telomere lengths, reduced telomerase activity and higher oxidative index)(ref). So the Telomere Shortening theory of aging impacts directly on the Suceptibility to Cardiovascular Disease theory. — In patients infected with HIV there is typically an initial period of several years during which the immune system is capable of controlling the disease before it finally breaks out into being full AIDS. There is evidence that during this period CD4 and CD8 cells reproduce at an abnormally high rate to keep up their battle with the infection. When these cell lines approach senescence and can no longer reliably reproduce because their telomeres are too short, they can no longer control the spread of the HIV virus and full AIDS finally breaks out. It is thought that enhanced activation of telomerase in these immune cells could make them essentially immortal and continuously capable of fighting off AIDS. Research progress towards this objective was reported recently by a UCLA/Geron team headed by Dr. Effros(ref). “The present study shows that exposure of CD8(+) T lymphocytes from HIV-infected human donors to a small molecule telomerase activator (TAT2) modestly retards telomere shortening, increases proliferative potential, and, importantly, enhances cytokine/chemokine production and antiviral activity.” Study of the Geron patent and literature references indicate that TAT-2 is cycloastragenol, a substance that can be derived through purification of astragaloside IV, itself a component of astragalus root. ” “In this study, we demonstrate that TAT2 can transiently activate telomerase, slow telomere loss, increase replicative capacity, and, importantly, enhance immune function in CD8+ T lymphocytes from HIV-1-infected persons. These data suggest a possible novel immune-based strategy to complement current treatments, which are primarily directed at the virus(ref).”
Part 2: The case for not bothering to take a telomerase extender supplement
There are several current research findings about telomeres and telomerase published in the last 18 months that go beyond those in the publications cited above. I believe they collectively suggest that it may not be worthwhile for normal people, even aging ones like myself, to take a telomerase extender. I cite some of this research and end the blog entry by commenting on my personal choice on this matter.
First of all, the association of telomere lengths with age is a very weak correlation applicable to populations but not necessarily to individuals.
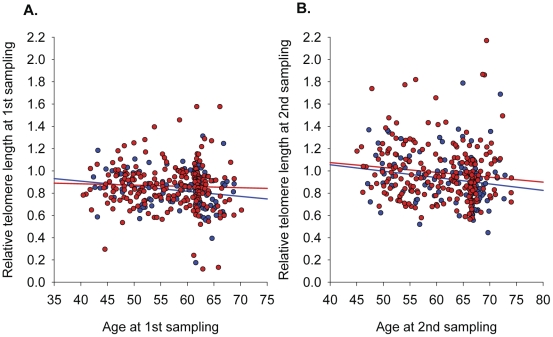
I start with this diagram from the April 2012 publication Leukocyte telomere length in the finnish diabetes prevention study:
The study on which this is based looked at 552 people in Finland with impaired glucose tolerance and the two samples of telomere lengths were made about 4.5 years about. The overall correlation of telomere lengths with age is very weak with an incredible scattering of individual values. Clearly, a significant number of older people had much longer telomeres than those in a significant portion of the younger people. If telomere length is a biomarkers of aging, it is an extremely poor one when it comes to individuals.
Second, from month-to-month and as people age and, telomere lengths can get longer as well as shorter. Inexorable telomere shortening due to cell division is simply not the case; the process is much more complicated than that.
For example, telomere elongation, as will be discussed further below, may be due to temporary constituent activation of telomerase or due to differentiation of stem and progenitor cells which have longer telomeres.
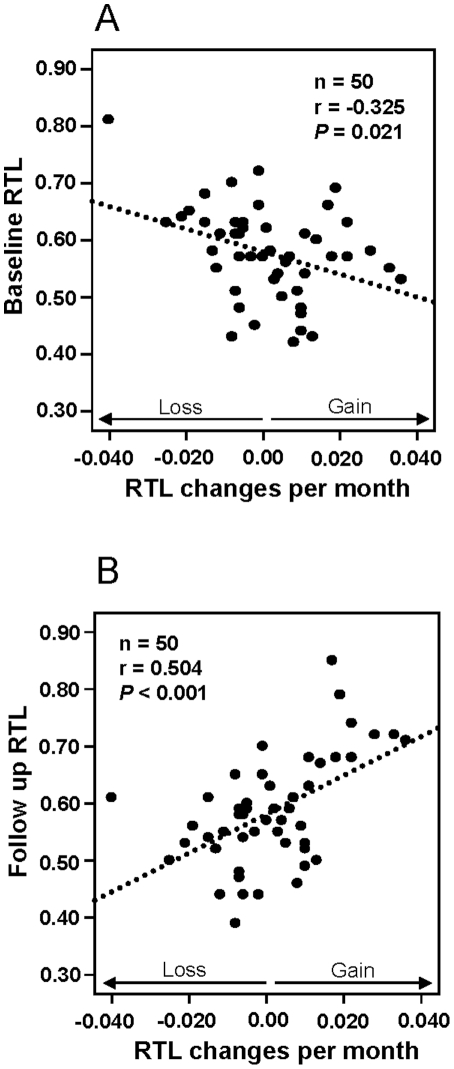
The “ticking clock” of telomere lengths is almost as likely to run backward as forward, making it a rather crummy clock. The 2011 e-publication Blood cell telomere length is a dynamic featurereports: “There is a considerable heterogeneity in blood cell telomere length (TL) for individuals of similar age and recent studies have revealed that TL changes by time are dependent on TL at baseline. TL is partly inherited, but results from several studies indicate that e.g. life style and/or environmental factors can affect TL during life. Collectively, these studies imply that blood cell TL might fluctuate during a life time and that the actual TL at a defined time point is the result of potential regulatory mechanism(s) and environmental factors. We analyzed relative TL (RTL) in subsequent blood samples taken six months apart from 50 individuals and found significant associations between RTL changes and RTL at baseline. Individual RTL changes per month were more pronounced than the changes recorded in a previously studied population analyzed after 10 years’ follow up. The data argues for an oscillating TL pattern which levels out at longer follow up times. In a separate group of five blood donors, a marked telomere loss was demonstrated within a six month period for one donor where after TL was stabilized. PCR determined RTL changes were verified by Southern blotting and STELA (single telomere elongation length analysis). The STELA demonstrated that for the donor with a marked telomere loss, the heterogeneity of the telomere distribution decreased considerably, with a noteworthy loss of the largest telomeres. In summary, the collected data support the concept that individual blood cell telomere length is a dynamic feature and this will be important to recognize in future studies of human telomere biology.” (Emphasis is mine,)
“Figure 1
Relative telomere length (RTL) and monthly RTL changes in the 6 month study.
Baseline RTL versus RTL changes per month, showing a significant negative correlation. Follow up RTL versus RTL changes per month, showing a significant positive correlation.”
The fact that telomere lengths in individuals may increase over years as well as decrease has been known for some time.
You can check out my blog entries The epigenetic regulation of telomeres and Lifestyle, dietary, and other factors associated with telomere shortening and lengthening.
The 2009 publication The individual blood cell telomere attrition rate is telomere length dependent reported “Age-associated telomere shortening is a well documented feature of peripheral blood cells in human population studies, but it is not known to what extent these data can be transferred to the individual level. Telomere length (TL) in two blood samples taken at approximately 10 years interval from 959 individuals was investigated using real-time PCR. TL was also measured in 13 families from a multigenerational cohort. As expected, we found an age-related decline in TL over time (r = -0.164, P<0.001, n = 959). However, approximately one-third of the individuals exhibited a stable or increased TL over a decade. The individual telomere attrition rate was inversely correlated with initial TL at a highly significant level (r = -0.752, P<0.001), indicating that the attrition rate was most pronounced in individuals with long telomeres at baseline. In accordance, the age-associated telomere attrition rate was more prominent in families with members displaying longer telomeres at a young age (r = -0.691, P<0.001). Abnormal blood TL has been reported at diagnosis of various malignancies, but in the present study there was no association between individual telomere attrition rate or prediagnostic TL and later tumor development. The collected data strongly suggest a TL maintenance mechanism acting in vivo, providing protection of short telomeres as previously demonstrated in vitro. Our findings might challenge the hypothesis that individual TL can predict possible life span or later tumor development.” (Emphasis is mine.)
The above-listed citation also makes the point that there is no clear association between pre-diagnostic telomere lengths, rate of telomere attrition and cancer disease susceptibility.
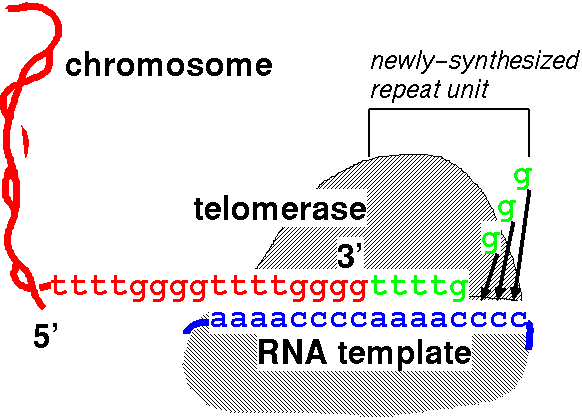
The idea that “telomerase lengthens telomeres by pasting ends of chromosomes back on” is vastly oversimplified.
The simplistic view:
Telomerase pastes telomeric DNA on the ends of chromosomes via a RNA template, making telomeres longer. Image source.
In fact, whether telomeres become longer or shorter at any point in any cell is the result of interaction of a multiplicity of factors including telomere proteins POT1, TRF1 and TRF2(ref)(ref), telomeric and subtelomeric methylation status(ref), other sheltrin subunits like TIN2, Rap1 and TPP1(ref)(ref), TERRA(ref), TANK1 AND TANK2, alternative lengthening mechanism (ALT)(ref)(ref), histone Dnmt and HDAC factors and many others – all in complex dynamic interaction. “Telomerase can also act as a transcriptional modulator of the Wnt-β-catenin signalling pathway and has RNA-dependent RNA polymerase activity(ref).”
A more complete description of the process involving many factors is shown in this diagram:
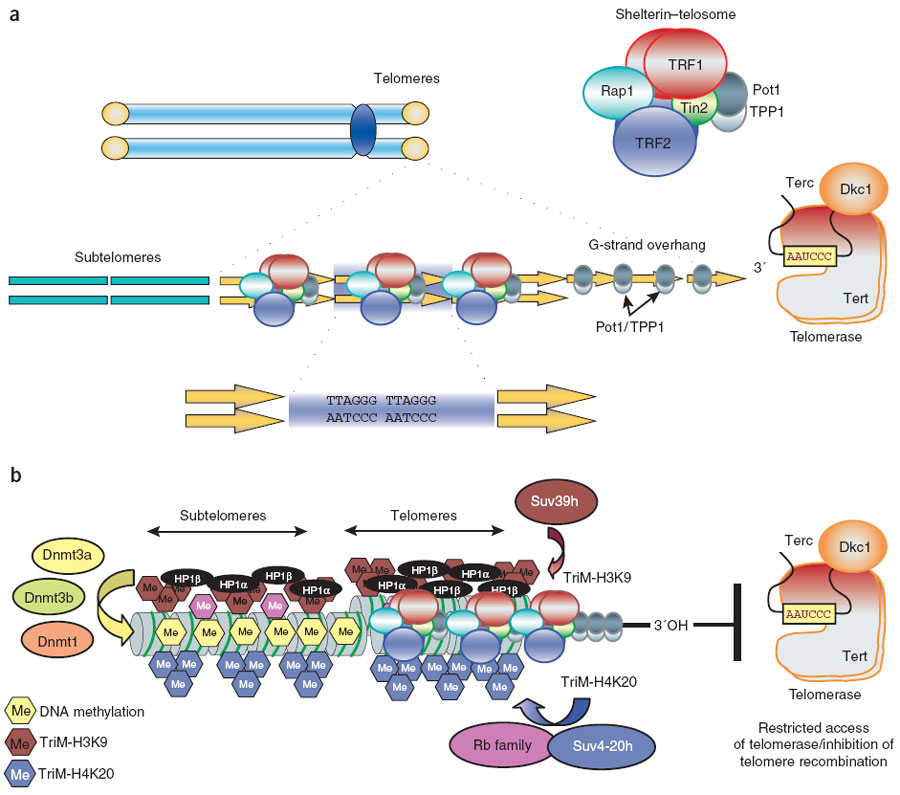 “(a) Mammalian telomeres consist of tandem repeats of the TTAGGG sequence that are bound by the shelterin–telosome protein complex. Adjacent to telomeres are the subtelomeric regions, which are also rich in repetitive DNA. (b) In addition to shelterin, mammalian telomeres also contain nucleosomes that show histone modifications characteristic of heterochromatin domains. In addition, subtelomeric DNA is heavily methylated. These chromatin modifications at telomeres and subtelomeres have been shown to negatively regulate telomere length and telomere recombination. TriM, trimethyl.” Image and legend from Telomere length, stem cells and aging.
“(a) Mammalian telomeres consist of tandem repeats of the TTAGGG sequence that are bound by the shelterin–telosome protein complex. Adjacent to telomeres are the subtelomeric regions, which are also rich in repetitive DNA. (b) In addition to shelterin, mammalian telomeres also contain nucleosomes that show histone modifications characteristic of heterochromatin domains. In addition, subtelomeric DNA is heavily methylated. These chromatin modifications at telomeres and subtelomeres have been shown to negatively regulate telomere length and telomere recombination. TriM, trimethyl.” Image and legend from Telomere length, stem cells and aging.
You can also have a look at the diagrams related to telomere extension in my 2011 blog entry The epigenetic regulation of telomeres.
Shortened telomeres is only one of a number of factors that can contribute to cellular senescence, and may often be a downstream effect of such factors.
Apostles of telomere-extending would lead us to believe that cell replication is the only or at least the main cause of cell senescence. This is not necessarily the case. “Several cellular stresses have been shown to induce a senescence-like growth arrest including shortened telomeres, DNA-damaging stresses, and drastic changes in chromatin structure, for example, through histone deacetylase (HDAC) induction(ref).” Also, overexpression of P16(Ink4a) has long been known to lead to cell senescence(ref). And removal of P16(Ink4a)-positive cells has been shown to restore a more-youthful phenotype(ref). See the 2009 publication Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. When cell senescence is indeed induced by telomere shortening the Smurf2 gene plays a key intermediary role in the process. See the blog entrySmurf2 in senescence, aging and diseases.
Pursuing a healthy lifestyle is probably a way to stabilize or increase telomere lengths.
Going back to the April 2012 publication Leukocyte telomere length in the finnish diabetes prevention study, “Leukocyte telomere length (TL) is considered a biomarker for biological aging. Shortened TL has been observed in many complex diseases, including type 2 diabetes (T2DM). Lifestyle intervention studies, e.g. the Diabetes Prevention Study (DPS), have shown a decrease in the incidence of T2DM by promoting healthy lifestyles in individuals with impaired glucose tolerance (IGT). Our aim was to study in the DPS the influence of the lifestyle intervention on TL. TL was measured by quantitative PCR-based method at two time points (N = 334 and 343) on average 4.5 years apart during the active intervention and post-intervention follow-up. TL inversely correlated with age. Our main finding was that TL increased in about two thirds of the individuals both in the intervention and in the control groups during follow-up; TL increased most in individuals with the shortest TL at the first measurement. — TL was not associated with development of T2DM, nor did lifestyle intervention have an effect on TL. No association between insulin secretion or insulin resistance indices and TL was observed. We did not detect an association between TL and development of T2DM in the DPS participants. It could be due to all participants being overweight and having IGT at baseline, both of which have been found to be independently associated with shorter leukocyte TL in some earlier studies. TL had no substantial role in worsening of glucose tolerance in people with IGT. Our study confirms that leukocyte TL can increase with time even in obese people with impaired glucose metabolism”
This publication suggests that, further, susceptibility to Type 2 diabetes appears to be independent of telomere lengths.
Telomeres in some cells tend to shorten with age; in other cells, age has no affect on telomere lengths.
The April 2012 publication Sustained telomere length in hepatocytes and cholangiocytes with increasing age in normal liver reports: “Aim: Telomeres, a validated biomarker of ageing, comprise multiple nucleotide repeats capping chromosomes that shorten with each cell cycle until a critical length is achieved, precipitating cell senescence. Only two previous studies studied the effect of aging in “normal” liver tissue, but were compromised by small sample size, limited age range, tissue derived from individuals with an increased risk of senescence and the use of liver homogenates. Method: We developed a robust large volume four-colour quantitative fluorescent in situ hybridisation (Q-FISH) technique to measure telomere length in large numbers of hepatocytes, Kupffer cells, hepatic stellate cells, CD4+ and CD8+ lymphocytes and cholangiocytes. Following validation against the gold standard (Southern blotting), the technique was applied to normal archived paraffin-embedded liver tissue obtained following reperfusion of implanted donor liver. We studied 73 highly selected donors aged 5 – 79 years with a short medical illness preceding death and no history of liver disease, reperfusion injury, or steatosis and normal graft function 1-year post transplant. Results: Cholangiocytes had significantly longer telomeres compared to all other intrahepatic lineages over a wide age range (p < 0.05). Age-related telomere attrition was restricted to sinusoidal cells (i.e. Kupffer (p = 0.0054) and stellate cells (p = 0.0001)). Cholangiocytes and hepatocytes showed no age-related telomere shortening. Conclusion: In normal liver and over a broad age range, cholangiocytes have longer telomeres than all other intrahepatic lineages. Age-related telomere length decline is restricted to Kupffer cells and stellate cells.”
In certain tissues for some cells, it is healthier to find shorter telomere lengths than longer ones.
The April 2012 publication Longer Leukocyte Telomere Length Is Associated with Smaller Hippocampal Volume among Non-Demented APOE ε3/ε3 Subjects provides a good example. “Telomere length shortens with cellular division, and leukocyte telomere length is used as a marker for systemic telomere length. The hippocampus hosts adult neurogenesis and is an important structure for episodic memory, and carriers of the apolipoprotein E ε4 allele exhibit higher hippocampal atrophy rates and differing telomere dynamics compared with non-carriers. The authors investigated whether leukocyte telomere length was associated with hippocampal volume in 57 cognitively intact subjects (29 ε3/ε3 carriers; 28 ε4 carriers) aged 49-79 yr. Leukocyte telomere length correlated inversely with left (r(s) = -0.465; p = 0.011), right (r(s) = -0.414; p = 0.025), and total hippocampus volume (r(s) = -0.519; p = 0.004) among APOE ε3/ε3 carriers, but not among ε4 carriers. However, the ε4 carriers fit with the general correlation pattern exhibited by the ε3/ε3 carriers, as ε4 carriers on average had longer telomeres and smaller hippocampi compared with ε3/ε3 carriers. The relationship observed can be interpreted as long telomeres representing a history of relatively low cellular proliferation, reflected in smaller hippocampal volumes. The results support the potential of leukocyte telomere length being used as a biomarker for tapping functional and structural processes of the aging brain.”
While telomere shortening seems to be involved in several disease processes, it is often not clear weather shortened telomeres are among the original driver causes of a serious disease processes or is a downstream effect. That is, shortened telomeres may be downstream consequences of other disease-causing chains of events, though, once drastically shortened, aberrant telomeres could then play a role in the disease process.
The November 2011 publication Telomere length in neoplastic and nonneoplastic tissues of patients with familial and sporadic papillary thyroid cancer reports: “Introduction: Many studies have found an association between altered telomerelength (TL), both attrition or elongation, and cancer phenotype. Recently, we have reported that patients with the familial form of papillary thyroid cancer (FPTC) have short telomeres in blood leucocytes. Aim: To evaluate relative TL (RTL) at somatic level in neoplastic and nonneoplastic tissues of patients with FPTC (n = 30) and sporadic PTC (n = 46). Methods: RTL was measured by quantitative PCR in neoplastic thyroid tissues, in the corresponding nontumor thyroid tissues (normal contralateral thyroid), and in other extrathyroidal tissues (lymph nodes, muscles, or buccal mucosa). RTL was also measured in adenomas and hyperplastic nodules. In a subset of samples, telomerase expression was measured by quantitative PCR. Results: Mean ± SD RTL of FPTC patients was short in neoplastic thyroid tissues (0.87 ± 0.2) with no difference from the normal contralateral thyroid tissues (0.85 ± 0.11) and extrathyroidal tissues (0.85 ± 0.31). On the contrary, in patients with sporadic PTC, the mean ± SD RTL in the neoplastic tissues (1.73 ± 0.63) was significantly shorter than that found in normal contralateral tissues (2.58 ± 0.89) and extrathyroidal tissues (2.5 ± 0.86). For all tissue samples (cancer, normal thyroid, and nonthyroidal tissues) the mean ± SD RTL of familial cases was shorter (P < 0.0001) than that found in tissues from sporadic PTC. RTL of FPTC was also lower (P < 0.0001) than that of 23 follicular adenomas (1.6 ± 0.7) and 24 hyperplastic nodules (2.2 ± 0.9). Conclusions: Our results demonstrate that short telomeres are a consistent feature of PTC, which in familial cases, is not restricted to the tumor tissue. This finding suggests that FPTC has a distinct, heritable, genetic background.” Whether shorter telomeres are causal of the disease or a consequence of it is unclear.
The December 2011 publication Short leukocyte telomere length is associated with aortic dissectionreports: “Background: Aortic dissection is an age-related and lethal vascular disease. Aging, which is associated with degeneration, is the major risk factor of aortic dissection. Telomeres are specialized DNA structures located at the end of eukaryotic chromosomes, the telomerelength could be considered as an index of vascular aging. The purpose of present study was undertaken to investigate the relationship between the leukocyte telomerelength and aortic dissection. Methods And Results: Seventy-two patients with aortic dissection and seventy-two sex- and age-matched subjects without vascular diseases were collected. Leukocyte telomerelength ratio (T/S ratio) was measured using a quantitative PCR method and analyzed. A significantly shorter leukocyte telomerelength in the patients with aortic dissection was found compared to the controls, [median 1.02 (interquartile range {IQR}:0.83-1.37) vs median 1.63 [IQR: 1.18-2.51), p<0.001]. The telomerelength in the control group showed a trend of inverse correlation with age (r=-0.226, p=0.056), however, there was no significant correlation in aortic dissection (r=0.062, p=0.607). The short leukocyte telomerelength was associated with aortic dissection, even after adjustment for other risk factor (OR=0.214, 95% CI: 0.085-0.537). Conclusion: Leukocyte telomerelength could be an independent predictor of aortic dissection. Measurement of the leukocyte telomerelength may be valuable for patients with a high risk of aortic dissection.” Again, whether shorter telomeres are causal of the disease or a consequence of it is unclear.
Yet another situation relating a disease process to telomere lengths is described in the April 2012 report Is the mean blood leukocyte telomere length a predictor for sporadic thoracic aortic aneurysm? Data from a preliminary study:“Telomeres have been postulated as a universal clock that shortens in parallel with cellular aging. They are specialized DNA-protein structures at the ends of chromosome with remarkable functions-preventing their recognition as double-stranded DNA breaks, protecting their recombination and degradation, and avoiding a DNA damage cellular response. Telomere shortening is currently considered the best aging marker, but is also a predictor for age-related diseases, including cardiovascular diseases. Biological age clearly seems to be a better predictor of vascular risk rather than chronological age. This concept is supported by key assumptions that peripheral blood leukocyte telomere content accurately reflects that of the vascular wall and its decrease is associated with premature vascular disease. Thus, we are analyzing whether the mean of blood leukocyte telomere length might also be a predictor for sporadic thoracic aortic aneurysm (S-TAA). The preliminary results seem to be promising. Shorter telomeres were detected in patients than in controls. Thus, mean of blood leukocyte telomere length could contribute to identify individuals at S-TAA risk.” Again, the observation is that in diseased patients, blood leukocyte telomere lengths tend to be shorter. And yet again, whether shorter telomeres are causal of the disease or a consequence of it is unclear.
Telomere lengths are also associated with stroke risk factors but the direction of causality is again unclear.
The April 2012 publication Leukocyte Telomere Length: A Focus on Cerebrovascular Eventsreports: “– The purpose of this study was to determine the associations between telomerelength and clinical and biological risk factors in ischemic stroke patients. A total of 215 stroke patients hospitalized in the Dijon, France, stroke unit were prospectively and continuously included from January to September, 2004. The telomerelength measured from peripheral blood leukocytes-leukocyte telomerelength (LTL)-was determined by real-time quantitative polymerase chain reaction. The results were compared with clinical and biological variables of interest collected at admission to find significant associations. Possible relationships between LTL and stroke subtypes were evaluated. A multiple regression that included all the variables significantly associated (p<0.20) with LTL in univariate analysis and age and subtypes of stroke confirmed a significant association with age (p<0.001), homocysteinemia (p=0,049), and levels of both antiphospholipid antibodies (p=0.019) and triglycerides (p=0.007). Linearity was verified and confirmed for each variable. The subtype of stroke did not significantly affect telomerelength. We were able to highlight significant associations between LTL and certain cerebrovascular risk factors in a general population of stroke patients. These associations did not depend on the ischemic stroke subtype.” The data was drawn from hospitalized stroke patients who of course had stroke risk factors. Again, there does not appear to be a basis for inferring whether shorter telomeres are causal of the disease process or a consequence of it.
Yet-another very recent study relating shorter telomere lengths to a disease process is described in the 2012 report Reduced telomere length in colorectal carcinomas: “Purpose: Telomeres play a key role in the maintenance of chromosome integrity and stability, and telomere shortening is involved in initiation and progression of malignancies. The aim of this study was to determine whether telomerelength is associated with the colorectal carcinoma. Patients and methods: A total of 148 colorectal cancer (CRC) samples and corresponding adjacent non-cancerous tissues were evaluated for telomerelength, P53 mutation, and cyclooxygenase-2 (COX-2) mutation detected by fluorescent immunohistochemistry. Telomerelength was estimated by real-time PCR. Samples with a T/S>1.0 have an average telomerelength greater than that of the standard DNA; samples with a T/S<1.0 have an average telomerelength shorter than that of the standard DNA. Results: Telomeres were shorter in CRCs than in adjacent tissues, regardless of tumor stage and grade, site, or genetic alterations (P=0.004). Telomerelength in CRCs also had differences with COX-2 status (P=0.004), but did not differ with P53 status (P=0.101), tumor progression (P=0.244), gender (P=0.542), and metastasis (0.488). There was no clear trend between T/S optimal cut-off values (<1 or > 1) and colorectal tumor progression, metastasis, gender, P53 and COX-2 status. Conclusion: These findings suggesting that telomere shortening is associated with colorectal carcinogenesis but does not differ with tumor progression, gender, and metastasis.”
Another recent relevant publications is(April 2012) Telomere maintenance mechanisms in malignant peripheral nerve sheath tumors: expression and prognostic relevance. In this case and in each case cited above, what is reported is simply an association of telomere shortening with a disease process, not that shortened telomeres were originally causative of the disease. I believe this is the general case.
Since diseases cause significant stress, accelerated immune system activity and cell turnover, I think it is not at all surprising to find shorter telomeres in diseased individuals. It is well-known that other forms of stress cause shortening of telomeres, independently of age. See for example the April 2012 report Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study, the May 2011 reportTelomere length and early severe social deprivation: linking early adversity and cellular aging, and the May 2012 report Telomere shortening in women resident close to waste landfill sites.
Shorter telomeres may serve to inhibit rather than promote emergence of cancers.
As Maria Blasco wrote, “Furthermore, mice that are simultaneously deficient in telomerase and the tumor suppressor proteins p19ARF, p16, p21, APC, ATM, DNA-PKcs, Ku, PARP1 and PMS2 also show reduced tumorigenesis67–73 (Table 1). This indicates that short telomeres are potent suppressors of cancer even in tumor-prone genetic backgrounds, most likely because telomere dysfunction induces cellular arrest and apoptosis66–73 — In turn, short telomeres impose a barrier on tumor development that can only be bypassed by abrogation of p53 or by TRF2 overexpression, which indicates that these molecular events are important in mediating cancer driven by short telomeres and chromosomal instability(ref).”
Oxidative stress may be a major cause of telomere attrition in. disease processes.
The April 2012 publication Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition reports: “Background: Abdominal aortic aneurysm (AAA) is a complex multi-factorial disease with life-threatening complications. AAA is typically asymptomatic and its rupture is associated with high mortality rate. Both environmental and genetic risk factors are involved in AAA pathogenesis. Aim of this study was to investigate telomerelength (TL) and oxidative DNA damage in paired blood lymphocytes, aortic endothelial cells (EC), vascular smooth muscle cells (VSMC), and epidermal cells from patients with AAA in comparison with matched controls. Methods: TL was assessed using a modification of quantitative (Q)-FISH in combination with immunofluorescence for CD31 or α-smooth muscle actin to detect EC and VSMC, respectively. Oxidative DNA damage was investigated by immunofluorescence staining for 7, 8-dihydro-8-oxo-2′-deoxyguanosine (8-oxo-dG). Results And Conclusions: Telomeres were found to be significantly shortened in EC, VSMC, keratinocytes and blood lymphocytes from AAA patients compared to matched controls. 8-oxo-dG immunoreactivity, indicative of oxidative DNA damage, was detected at higher levels in all of the above cell types from AAA patients compared to matched controls. Increased DNA double strand breaks were detected in AAA patients vs controls by nuclear staining for γ-H2AX histone. There was statistically significant inverse correlation between TL and accumulation of oxidative DNA damage in blood lymphocytes from AAA patients. This study shows for the first time that EC and VSMC from AAA have shortened telomeres and oxidative DNA damage. Similar findings were obtained with circulating lymphocytes and keratinocytes, indicating the systemic nature of the disease. Potential translational implications of these findings are discussed.”
Induction of the stress hormone cortisol could also lead to shorter telomeres. From my treatise: “Research reported by Rita Effros of UCLA and her colleagues(ref) indicates that cortisol inhibits the expression of telomerase in immune system cells, This explains why people subject to considerable stress tend to have shorter telomeres. Of course, cortisol is produced in the body in response to stress.”
Mutations in the telomerase gene are linked to shorter telomere lengths and certain disease susceptibilities or disease processes. There is no question that such mutations are causative of certain rare diseases.
The May 2012 publication hTERTCancer Risk Genotypes Are Associated With Telomere Lengthreports: “Telomere biology is associated with cancer initiation and prognosis. Collected data suggest that blood cell telomerelength (TL) can change over time, which may be related to development of common disorders, such as cardiovascular diseases and cancer. Recently, single nucleotide polymorphisms in the region of the human telomerase reverse transcriptase (hTERT) gene were associated with various malignancies, including glioma, lung and urinary bladder cancer, and telomerase RNA gene hTERC genotypes were recently linked to TL. In the present study a hypothetical association between identified genotypes in hTERT and hTERC genes and TL were investigated. We analyzed 21 polymorphisms, covering 90% of the genetic variance, in the hTERT gene, two genetic variants in hTERC, and relative TL(RTL) at average age 50 and 60 in 959 individuals with repeated blood samples. Mean RTL at age 60 was associated with four genetic variants of the hTERT gene (rs2736100, rs2853672, rs2853677, and rs2853676), two of which reported to be associated with cancer risk. Two alleles (rs12696304, rs16847897) near the hTERC gene were confirmed as also being associated with RTL at age 60. Our data suggest that hTERT and hTERC genotypes have an impact on TL of potential relevance and detectable first at higher ages, which gives us further insight to the complex regulation of TL.”
The classical 2007 document by Maria Blasco Telomere length, stem cells and agingstated: “Telomere shortening occurs concomitant with organismal aging, and it is accelerated in the context of human diseases associated with mutations in telomerase, such as some cases of dyskeratosis congenita, idiopathic pulmonary fibrosis and aplastic anemia. People with these diseases, as well as Terc-deficient mice, show decreased lifespan coincidental with a premature loss of tissue renewal, which suggests that telomerase is rate-limiting for tissue homeostasis and organismal survival.
The April 2012 report Genetic polymorphisms in telomere pathway genes, telomere length, and breast cancer survival similarly implicates defects in telomerase pathway genes with survivability in breast cancer. “The impact of genetic variants in telomere pathway genes on telomerelength and breast cancer survival remains unclear. We hypothesized that telomerelength and genetic variants of telomere pathway genes are associated with survival among breast cancer patients. A population-based cohort study of 1,026 women diagnosed with a first primary breast cancer was conducted to examine telomerelength and 52 genetic variants of 9 telomere pathway genes. Adjusted Cox regression analysis was employed to examine associations between telomerelength, genetic variants and all-cause and breast cancer-specific mortality. Longer telomerelength was significantly correlated with all-cause mortality in the subgroup with HER-2/neu negative tumors (HR = 1.90, 95 % CI: 1.12-3.22). Carrying the PINX1-33 (rs2277130) G-allele was significantly associated with increased all-cause mortality (HR = 1.45, 95 % CI: 1.06-1.98). Three SNPs (TERF2-03 rs35439397, TERT-14 rs2853677, and TERT-67 rs2853669) were significantly associated with reduced all-cause mortality. A similar reduced trend for breast cancer-specific mortality was observed for carrying the TERT-14 (rs2853677) T-allele (HR = 0.57, 95 % CI: 0.39-0.84), while carrying the POT1-18 (rs1034794) T-allele significantly increased breast cancer-specific mortality (HR = 1.48, 95 % CI: 1.00-2.19). However, none of the associations remained significant after correction for multiple tests. A significant dose-response effect was observed with increased number of unfavorable alleles/genotypes (PINX1-33 G-allele, POT1-18 T-allele, TERF2-03 GG, TERT-14 CC, and TERT-67 TT genotypes) and decreased survival. These data suggest that unfavorable genetic variants in telomere pathway genes may help to predict breast cancer survival.”
See also the 2009 publication A spectrum of severe familial liver disorders associate with telomerase mutations
Some researchers observing shorter telomeres in diseased patients than in non-diseased ones see shorter telomeres as a “risk factor” for the disease.
In all fairness, a few publications take this viewpoint, though I think it is faulty logic, like listing baldness as a risk factor for aging or catching fish as a risk factor for fishing. They are influenced by a historic (1990s) viewpoint that shorter telomeres are a major cause of diseases, a viewpoint that I believe remains largely unproven. An example is the April 2012 publication Shorter telomere length is associated with increased ovarian cancer risk in both familial and sporadic cases: “Background: Alterations in telomere maintenance mechanisms leading to short telomeres underlie different genetic disorders of ageing and cancer predisposition syndromes. It is known that short telomeres and subsequent genomic instability contribute to malignant transformation, and it is therefore likely that people with shorter telomeres are at higher risk for different types of cancer. Recently, the authors demonstrated that the genes BRCA1 and BRCA2 are modifiers of telomerelength (TL) in familial breast cancer. The present study analysed TL in peripheral blood leucocytes of hereditary and sporadic ovarian cancer cases, as well as in female controls, to evaluate whether TL contributes to ovarian cancer risk.MethodsTL was measured by quantitative PCR in 178 sporadic and 168 hereditary ovarian cases (46 BRCA1, 12 BRCA2, and 110 BRCAX) and compared to TL in 267 controls. Results: Both sporadic and hereditary cases showed significantly shorter age adjusted TLs than controls. Unconditional logistic regression analysis revealed an association between TL and ovarian cancer risk with a significant interaction with age (p<0.001). Risk was higher in younger women and progressively decreased with age, with the highest OR observed in women under 30 years of age (OR 1.56, 95% CI 1.34 to 1.81; p=1.0×10(-18)). Conclusion: These findings indicate that TL could be a risk factor for early onset ovarian cancer.” The researchers looked at cases of ovarian cancer and healthy controls and found, like in so many other studies, that the cancer patients had shorter telomeres. I see no basis whatsoever in this research for concluding that healthy women with shorter telomeres are at greater risk for ovarian cancer.
Telomere length is not a particularly good biomarker for predicting the life expectancy of the oldest old.
The March 2012 publication Telomere length, comorbidity, functional, nutritional and cognitive status as predictors of 5 years post hospital discharge survival in the oldest oldreports: “Background: Telomerelength has been considered in many cross-sectional studies as a biomarker of aging. However the association between shorter telomeres with lower survival at advanced ages remains a controversial issue. This association could reflect the impact of other health conditions than a direct biological effect. Objective: To test whether leukocyte telomerelength is associated with 5-year survival beyond the impact of other risk factors of mortality like comorbidity, functional, nutritional and cognitive status. Design: Prospective study. Setting and participants: A population representative sample of 444 patients (mean age 85 years; 74% female) discharged from the acute geriatric hospital of Geneva University Hospitals (January-December 2004), since then 263 (59.2%) had died (December 2009). Measurements: Telomerelength in leukocytes by flow cytometry. Results: In univariate model, telomerelength at baseline and cognitive status were not significantly associated with mortality even when adjusting for age (R2=9.5%) and gender (R2=1.9%). The best prognostic predictor was the geriatric index of comorbidity (GIC) (R2=8.8%; HR=3.85) followed by more dependence in instrumental (R2=5.9%; HR=3.85) and based (R2=2.3%; HR=0.84) activities of daily living and lower albumin levels (R2=1.5%; HR=0.97). Obesity (BMI>30: R2=1.6%; HR=0.55) was significantly associated with a two-fold decrease in the risk of mortality compared to BMI between 20-25. When all independent variables were entered in a full multiple Cox regression model (R2=21.4%), the GIC was the strongest risk predictor followed by the nutritional and functional variables. Conclusion: Neither telomeres length nor the presence of dementia are predictors of survival whereas the weight of multiple comorbidity conditions, nutritional and functional impairment are significantly associated with 5-year mortality in the oldest old.” This is not a surprising result given the fact graphically illustrated above that many very old people have very long telomeres.
There is much more to be said about telomere biology which is quite complex. Many dietary phytochemicals and supplements have been shown to have telomere-extending capabilities, quite apart from specific expensive proprietary supplements explicitly marketed because they can presumably extend telomeres.
I have made these points in my treatise and in several previous blog entries. From my treatise: “The January 2010 blog post Vitamins, supplements and telomerase – upregulation or downregulation? points to a different study in which telomere lengthening was observed over a long period of time for a sizeable portion of the population studied. Also, it appears that taking a number of popular supplements in the anti-aging firewalls supplement regimen like Vitamin E, fish oils, Vitamin D3 and resveratrol can lead to telomeres being longer than they otherwise might be, possibly because they induce the production of telomerase, possibly for other reasons. And, several of these supplements actually turn off telomerase in cancer cells. — These results suggests to me that telomere shortening is a complex process involving a balance of shortening due to cell division, lengthening due to natural telomerase expression and perhaps cell replacement due to differentiation of stem cells. And these in turn are affected by many lifestyle and dietary factors and moderated by cell-signaling feedback loops.”
From The epigenetic regulation of telomeres. “Although this blog entry focuses on epigenetic regulation as a new and very important aspect of telomere biology, I continue to stand behind what I have written related to telomeres and telomerase reflecting a shift over a three-year period. The most-recent relevant blog entries were written in October 2010: Telomere lengths, Part 3: Selected current research on telomere-related signaling, telomere lengths, cancers and disease processes, Part2: lifestyle, dietary, and other factors associated with telomere shortening and lengthening, and Part1: telomere lengths, cancers and disease processes. These entries contain a great deal of information as well as links to multiple earlier blog entries on telomeres and telomerase. And, of course, the 12thth theory of aging discussed in my treatise is Telomere Shortening and Damage. Three years ago, I thought that taking astragaloside IV or cycloastragenol supplements to extend telomeres was possibly a good anti-aging intervention. I no longer see that as the case.”
Telomeres and telomerase remain important and thriving areas of research. This blog entry presents a sample of research, much of it very recent (March – May 2012), intended to debunk many of the simplistic myths associated with the telomere-shortening theory of longevity and the marketing of expensive telomere-extending supplements.
As I said before(ref) “ Telomere length homeostasis is a devilishly complex topic and we are just starting to sort out all the factors and interactions involved. CHIP, HSP90 and p23 get added to TRF1/TRF2, shelterin-complex, PinX, Apollo, TERRA, ORC, HP1, H3 K9me3 and tankyrase as factors involved in telomerase extension/shortening. And of course a host of lifestyle and dietary measures are involved. Gone are the old days of simple thinking “Want longer telomeres? Just activate your telomerase gene.”
Finally and of central importance, it is not at all clear that telomere lengths are predictive of human lifespan or death from any particular underlying cause like infectious diseases, cancer, or cardiac or cerebrovascular diseases.
Many people who take telomere -extending supplements are motivated because they believe that having longer telomeres will increase the probability that they will live longer. However, the research evidence related to this proposition is contradictory and on the whole inconclusive.
The 2012 publication Telomeres in disease reports: “Telomeres and aging: Telomeres shorten as we age. Consequently, telomere length has been postulated as a marker of “genetic age” (mitotic clock), as a fundamental explanation for the aging process, and has been marketed as a simple predictor of longevity. Telomere length assays have been bundled with recommendations for lifestyle modification and for drug therapy, neither based on appropriate clinical studies. Simple but appealing arguments relating telomeres and aging may be viewed, with some skepticism, as currently controversial, likely simplistic, and potentially harmful. Telomere length indeed reflects the cell’s past proliferative history and future propensity to apoptosis, senescence, and transformation. However, cellular aging is not equivalent to organ or organismal aging. — There are several considerations in relating telomere biology to aging. First, physiologically there is overlap between the shortest telomere length of young children and the longest telomeres of the elderly. Most telomere shortening occurs early in life, in association with growth, and when the rate of disease in general is low. The paradigmatic telomere syndrome of dyskeratosis congenita is not at all typical of the progerias, inherited syndromes in which patients not only appear old but suffer diseases of aging, like premature atherosclerosis or dementia. Conversely, the organ damage of dyskeratosis congenita is not very similar to aging of marrow, lungs, and liver. The marrow becomes mildly hypocellular in older individuals but stem cell numbers may actually increase and blood counts remain stable; neither the liver nor lung normally become fibrotic with advanced age. Although relatively short telomeres of leukocytes have been associated with cardiovascular events among adults, the clinical correlations have not been consistent, and they may be related to overall reactive oxygen species exposure. — Studies in humans have attempted to relate short telomeres to longevity. In the provocative initial publication from the University of Utah, individuals around 60 years of age who had the longest telomeres lived longer than did subjects with the shortest telomeres, but the most associated cause of death in the latter group was, inexplicably, infection, and those with shorter telomeres did not have a higher rate of cancer deaths [19]. Heart disease as the cause of death was also more common in subjects with the shortest telomeres. Subsequent studies have produced conflicting findings. The Cardiovascular Health Study of subjects over 65 years of age found that individuals in the shortest quartile for telomere length were 60% more likely to die than those in the longest quartile [20]. Causes of death related to short telomeres were again infectious. Two twin studies at older age also correlated shorter telomeres with poorer survival [21,22]. Finally, an Italian cohort study that looked at participants at time zero and after 10 years found that death within 10 years was significantly more common in those with shorter telomeres [23]. — In contrast, these associations have not been confirmed in other studies of older subjects. Blackburn and Cawthon reported that telomere length failed to predict survival, but correlated with years of healthy life [24]. In a Danish study of people aged 73 to 101 years, telomeres correlated with life expectancy in simple univariate analysis but, when corrected for age, did not predict longevity [25]. In Dutch men with a mean age of 78 years, telomere length eroded with aging but failed to correlate with mortality [26]. In a Finnish investigation, telomere length did not predict overall mortality [27]. Finally, in an analysis from California, short telomere length predicted death from cardiovascular disease in women but not in men, where the rate of shortening predicted mortality rather than length itself [28]. The discrepancies in these results may have several sources. In some analyses, telomere lengths may have been studied as a surrogate marker of age. In addition, retrospective studies may uncover “positive” associations that are random and not reproduced in follow-up investigations.” (Emphasis is mine.)
The report goes on: “The telomere hypothesis of aging has also been tested in mice. For instance, in a murine model of telomerase deficiency and accelerated telomere attrition, researchers found that low telomerase expression deregulated certain intracellular pathways involved in mitochondrial function and glucose metabolism, ultimately causing heart muscle disease [29]. Interestingly, telomerase reactivation in these mice restored glucose production and heart function. However, the abnormalities observed in telomerase-deficient animals did not resemble those typical of humans with very short telomeres, in whom heart disease is rare. Translation of mouse experiments on telomeres to human physiology and disease should be approached with caution. Mice are not the ideal model for telomere attrition and its effects on aging: murine telomeres are 5 to 10 times longer than in humans, in spite of their much shorter lifespan. When telomerase is “knocked out” in mice, they live a healthy life for several generations; even though late generation animals with very short telomeres are infertile, they do not display the clinical phenotypes characteristic of human telomeropathies (bone marrow failure, pulmonary fibrosis, hepatic cirrhosis). Also, telomerase-deficient mice do not have a higher incidence of cancer, unless the p53 gene also is down modulated, in contrast to humans with telomerase deficiency, whom are at very high risk of developing cancer.”
The 2009 study Association Between Telomere Length, Specific Causes of Death, and Years of Healthy Life in Health, Aging, and Body Composition, a Population-Based Cohort Study, participated in by a prestigious team including Elizabeth H. Blackburn reports: “Although telomere length (TL) is known to play a critical role in cellular senescence, the relationship of TL to aging and longevity in humans is not well understood. In a large biracial population-based cohort, we tested the hypotheses that elderly persons with shorter TL in peripheral white blood cells have poorer survival, shorter life span, and fewer years of healthy life (YHL). Associations were evaluated using Cox proportional hazard models and linear regression analyses where appropriate. TL (in kilo base pairs) was not associated with overall survival (hazard ratio 1.0; 95% confidence interval 0.9–1.1) or death from any specific underlying cause including infectious diseases, cancer, or cardiac and cerebrovascular diseases. TL, however, was positively associated with more YHL (β = 0.08 ± 0.04, p = .03). Findings suggest that TL may not be a strong biomarker of survival in older individuals, but it may be an informative biomarker of healthy aging.” (Emphasis is my own.)
My personal choice regarding taking explicit astragalus-based telomerase-activating substances.
I stopped taking them over 2 years ago and don’t now plan to resume taking them because:
- The research such as that cited above related to the usefulness of such substances for retarding diseases or aging seems relatively weak and largely unsupported for humans when compared to research in other areas, such as often reported in this blog, examples being Nrf2 and antioxidants, mTOR, IGF-1, MAPK, AMPK, PGC-1α and the SIRTs. Solid research suggests that such other pathways are more critical for health and longevity than the telomere-related ones.
- There is the question of cost. When the Patton Protocol was initiated a few years back, its cost including TA-65® was $25,000 a year. Now an unbundled month’s supply (30 caps) of TA-65® purchased from Revgenetics costs $217. This is a lot cheaper but still more than 6 times as much as any other supplement I am taking and would create a significant yearly cost for me.
- A number of the very-important supplements I take daily like resveratrol and curcumin with very well-documented health benefits reportedly interfere with the effectiveness of the astragalus-based supplements. So people taking the astragalus-based supplements either give these other supplements up or take them every other day. I think this is an extremely poor tradeoff when it comes to highly-researched and well- known benefits for health and longevity – even if the extender supplement was cost-free. Pubmed.org lists 2 research publications relevant to TA-65 (both covered above), 4735 related to curcumin and 4677 related to resveratrol.
- Finally I do not need to take the astragalus-based supplements to get the result of making sure my telomeres are long. A number of lifestyle interventions I am pursuing, dietary substances I consume, and phytosubstance supplements I take are correlated with very effectively enlongating telomeres (ref)(ref)(ref)(ref).


Timely write up on this topic.
Bruno Bernardes de Jesus et al., Telomerase gene therapy in adult and old mice delays ageing and increases longevity without increasing cancer, EMBO Molecular Medicine, 2012 DOI: 10.1002/emmm.201200245
To soon to tell if this would work with humans and if refinements (Better 1 time dose or multiple doses) could lead to longer lifespan. How might this interact with other emerging lifespan increasing methods?
Lower Protein, EOD or Caloric Restriction, Mannoheptulose, buckyballs etcetera
Eric 25001:
Excellent questions. See my other comment posted here. What a wonderfully rich game we are playing, and with such magnificant stakes! We need to keep tuned.
Vince
Hello Vincent, Why everybody forget the most robust and 100% scientifically proven method of significant (by 40-50% life extension) using caloric restriction and periodic fasting. We are doing such anti-aging programs in Europe: http://www.anti-aging-plan.com It is not my advertising – it is just for cleaning your mind and ask you to do a fresh look on the anti-aging CR paradigma. And look for some elixir drug – is naive. All the best, Arcady Economo, pharmacologist, gerontologist.
Spot ON… Arcady, SPOT ON!
Hello and thank you very much for this comprehensive article.
I came across this (EMBO Molecular Medicine 2012, May 15th) study (‘did not read the original paper) in mice and I guess you too. http://onlinelibrary.wiley.com/doi/10.1002/emmm.201200245/abstract
By the age of two years inducing telomerase through a viral vector increased lifespan by 13 % along with physiologic improvement and last not least no increase in cancer.
best
Markus
Oh well sorry
sorry. I overlooked that eric25001 already posted the study.
Markus
Markus
This is actually a very interesting study since it establishes life extension in genetically normal animals due to telomerase induction. A somewhat more comprehensive writeup of it is at http://onlinelibrary.wiley.com/doi/10.1002/emmm.201200246/full
Mice normally die with fairly long telomeres so it is interesting that the approach actually extends life and healthspans of the rodents. As speculated I suspect this is due to longer leases on life of adult stem cells.
The approach might well work in humans and could possibly produce excellent results, but it is likely to be a long time before we find out whether this is so. Researchers are very leary of treatments that modify the human genome, and human gene therapy approaches are very few and far in-between. The approach used, Gene therapy via recombinant adeno-associated virus induction vectors, is one that worries us because the vectors could induce cancers. And again, the added hTERT genes could be oncogenic. Hopefully there will be studies on higher animals and eventually simians that check the approach out in the medium term. Thanks for the link.
Vince
Here is a study that may or may not tie in with telomerase. However it seems restricting ones feeding time to 8 hour time periods would work with many diets talked about like protein or calorie restriction. One common factor seems the uptick in autophagey. The impact for humans IF this could increase health and reduce weight by eating the same calories but in an 8 hour window could be simple and have higher compliance than diet calorie restrictions. I would think lifespans would be increased. Eric
Megumi Hatori, Christopher Vollmers, Amir Zarrinpar, Luciano DiTacchio, Eric A. Bushong, Shubhroz Gill, Mathias Leblanc, Amandine Chaix, Matthew Joens, James A.J. Fitzpatrick, Mark H. Ellisman, Satchidananda Panda. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metabolism, 2012; DOI: 10.1016/j.cmet.2012.04.019
Eric25001
I will have a look. Have you seen Victor’s post Alternate-day Fasting – a better alternative at http://www.anti-agingfirewalls.com/2012/01/14/alternate-day-fasting-%e2%80%93-a-better-alternative/ ?
Thanks for the tip.
Vince
Yes
That is why I thought this study would be of interest.
Based on this article I have started to put my eating day inside an 8 hour window. Seems a small change from a 12 to 16 hour window. Time will tell.
The article shows a number of improvements in the results of the time restricted animals. Eric
http://www.sciencedirect.com/science/article/pii/S1550413112001891#app2
Another recent study finding positive results from IF, but even better results when polyphenols are included. The link at the bottom is to the full text.
http://extremelongevity.net/2012/05/18/polyphenols-increase-caloric-restriction-induced-lifespan-extension/
Jeg3 and Eric25001
Thanks for the reference tips. I will look into them. Unfortunately in the family and social-intensive world in which I live, it would be extremely difficult for me personally shift my eating habits to an 8 hour window or alternative day schedule. Meals are an intrinsic part of my social lifestyle. I can and do add plenty of polyphenols though and will keep up with the research. More and more and more to follow.
Vince
Regarding the use of natural compounds to activate telomerase, or to purportedly suppress it…
The world patent application on product B has finally been made available to the public. You can find the text here.
http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012106692&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
The invention description section of this patent application contains a wealth of previously unpublished information on newly discovered phytochemicals that activate telomerase.
The key ingredient in the original version of product B (the version at the time of the patent filing) is silymarin / milk thistle. It seems that version1 of product B was ~50% silymarin / milk thistle by weight.
Quoting from section 0028 of the patent:
“[0028] Based on the test results, silymarin is identified as a significant potential telomerase inducer. Regular ingestion by an individual of effective amounts of silymarin extracted from milk thistle is expected to reverse telomere shortening in the individual and produce longer telomeres.”
It appears that the telomerase activating capability of silymarin varies quite a bit with how the silymarin is extracted and/or prepared. Several examples are listed in the patent. You can find my comments on this here:
http://www.longecity.org/forum/topic/50995-product-b-telomerase-activation/page__st__450__p__531252#entry531252
The patent goes on to list many other telomerase activating compounds identified in the Sierra Sciences RT-PCR screen. The combination of many of these other natural componds comprise much of the remaing 50% of product B by weight.
Quoting from section 0026 of the patent:
[0026] Follow-on testing of additional samples was undertaken using the same methodology. Hits were obtained on samples containing by horny goat weed (Epimedium sagittatum), Grape Seed (Vitis vinifera), Turmeric (Curcuma longa), Bacopa (Bacopa monnieri), Pomegranate (Punica granatum), DL-alpha lipoic acid, Asian ginseng, (Panax ginseng), Green Tea, White Tea, Black Tea (Camellia sinensis), Acacia (Acacia nilotica), Plantain (Plantago major), L-glutathione, Velvet Bean (Mucuna pruriens), Hawthorn (root) (Crataegus pinnatifida), Quercetin, Boswellia, (Boswellia serrata), Maca (Lepidium meyenii), Hawthorn (fruit) (Crataegus pinnatifida), Resveratrol, Harada (Terminalia chebula), Shilajit, Chia (Salvia hispanica), N-Curcusorb (trade name for version of Turmeric), Polygonum Cuspidatum (trans resveratrol), pterostibene, (a synthetic form of resveratrol developed by ChronaDex Company), Tumipure (trade name for Turmeric ingredient by Naturex Company).
There are dozens of publications suggesting that these same natural compounds listed above suppress telomerase in various cancer cell lines, including silymarin itself. I believe this patent powerfully supports a point I made earlier in the comment section of this blog.
http://www.anti-agingfirewalls.com/2009/06/11/do-resveratrol-curcumin-and-egcg-from-green-tea-really-inhibit-the-expression-of-telomerase/#comment-56728
I resummarize the main point here: The mechanisms involving the induction/suppression of the telomerase enzyme in a cancer cell and in a healthy somatic cell are quite different. The mechanism in a cancer cell is messy, complictaed, and poorly defined/understood. In a cancer cell, telomerase is induced by a bird’s nest of accumulated mutations — unique to every indivdual cancer cell. The mechanism in a healthly somatic cell is realatively well-defined and reasonably understood — and is essentially the same in all somatic cell types. The molecular biology is such that the same compound can BOTH suppress telomerase in cancer cells, yet induce it in healthy somatic/stem cells. When you take the various natural compounds listed in this patent, you are probably getting the best of both worlds — both preventing cancer (by suppressing telomerase in cancer cells, as well as thru many other mechanisms) and activating telomerase at the same time in healthy somatic/stem cells!
What a powerful confirmation of the health benefits of the supplements many of us have been taking for years, inlcuding Vince’s firewalls program: Is nature not amazing!
All the best,
Louis
I think there are many studies that prove it
Hi Vincent,
What supplements/herbs do you recommend to take as anti aging? I am an 33 years old man.
Thanks!
Hi Danutz
“Recommend” is too strong a word given the uncertainties associated with biology; I prefer “suggest.” Having said that:
1. A short list of my “top 10 supplements” suitable for a younger person like yourself in good health can be found at http://www.vincegiuliano.name/10topsupplements.htm
I put this list together in 2009 and it still looks pretty good to me.
2. A much more comprehensive list perhaps suitable for an old geeze like me in good health is in my treatise ANTI-AGING FIREWALLS – THE SCIENCE AND TECHNOLOGY OF LONGEVITY at http://www.vincegiuliano.name/Antiagingfirewalls.htm Look for the Table entitled TABLE I – SUPPLEMENTS IN COMBINED FIREWALLS.
Vince
Pingback: Mitochondria Part 2: Mitochondrial Responses to Stress: Mitochondrial Signaling: Survival and Death Pathways | AGING SCIENCES – Anti-Aging Firewalls
Pingback: URL