By Vince Giuliano
Small doses of radiation, such as from occasional X-rays or living at a high altitude, may actually be good for you according to the radiation hormesis hypothesis discussed here. Although this hypothesis is about 30 years old now, its history has been controversial and related to political issues such as the safety of nuclear energy. The hypothesis has not been embraced by those concerned with radiation safety in X-ray applications, nuclear plants and space travel. Recent research lends strong support to the hypothesis however, and characterizes the biological pathways through which it works. This blog entry briefly reviews the concept of hormesis and looks at a representative sample of hundreds of research articles related to radiation hormesis.
About hormesis
I introduced the concept of hormesis in this blog in November 2009. My blog posting Hormesis and age retardation describes hormesis as a process of “challenging cells and body systems by mild stress resulting in them becoming stronger and resistant to aging(ref). The stress can be physical, chemical and even possibly psychological.” Exercise is an example. That blog entry reviews the science behind hormesis and some of its demonstrable anti-aging effects. Also, see my blog entry Stress and longevity for further discussion of how moderate stresses confer longevity.
From Wikipedia: “Hormesis (from Greek hórmēsis “rapid motion, eagerness,” from ancient Greek hormáein “to set in motion, impel, urge on”) is the term for generally favorable biological responses to low exposures to toxins and other stressors. A pollutant or toxin showing hormesis thus has the opposite effect in small doses as in large doses. — In toxicology, hormesis is a dose response phenomenon characterized by a low dose stimulation, high dose inhibition, resulting in either a J-shaped or an inverted U-shaped dose response. — The hormesis model of dose response is vigorously debated.[1] The notion that hormesis is a widespread or important phenomenon in biological systems is not widely accepted.[2] — The biochemical mechanisms by which hormesis works are not well understood. It is conjectured that low doses of toxins or other stressors might activate the repair mechanisms of the body. The repair process fixes not only the damage caused by the toxin, but also other low-level damage that might have accumulated before without triggering the repair mechanism.” 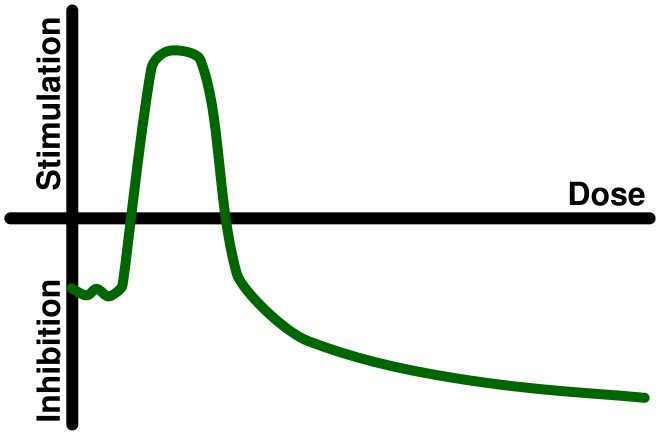 |
A good review of the current status of hormesis is provided in the 2011 e-publication Hormesis pervasiveness and its potential implications for pharmaceutical research and development. “ The volume of literature published in this area each year has grown 10-fold in large part due to single, original papers in a variety of high impact factor, scientific, peer-reviewed international journals, but also due to entire volumes of journals which have been devoted to hormesis. Some of the various areas include aging, benign prostate enlargement, biochemical and physiological cellular responses, caloric restriction, cardiovascular function, cancer and tumor development, chemo-sensitization, chemotherapy, dermatology, drug binding, hair growth, sexual dysfunction, ocular diseases, osteoporosis, oxidative stress, prion diseases and synaptic plasticity (Calabrese 2008a, Maynard et al. 2008). Four special volumes concentrated on neuroscience, including neuronal survival, neurite outgrowth, glial adaptive responses to neurotoxins, p-glycoprotein efflux transporter activity, anxiety and anxiolytic drugs, epilepsy, traumatic brain injury, stroke, addiction, memory and Alzheimer’s Disease (Calabrese 2008b).”
Historically, many scientists believed that hormesis, or “a little stress is a good thing,” was somewhat of a magical belief not fully to be trusted despite evidence supporting it. As evidence for hormesis builds up, however, that skepticism may be slowly dissolving. As pointed out below, I believe it is no longer true that “ The biochemical mechanisms by which hormesis works are not well understood.”
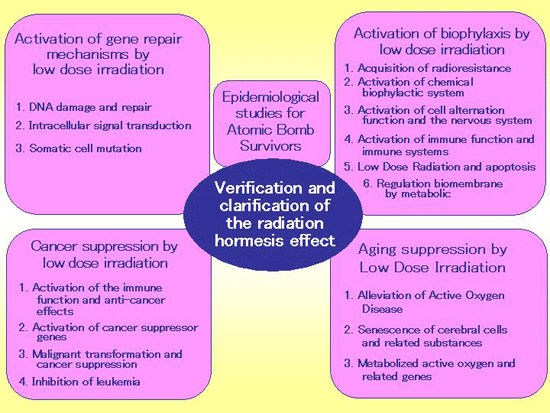
Radiation hormesis, though still controversial, is actually an old topic.
It has been studied in Japan since 1982 in multiple research centers. The Nuclear Technology Research Center there has published a historical timetable of early events.
Among the early studies from Japan, a 1993 publication even went so far as to suggest that low dose radiation might be used as a protective measure to prevent lung cancer metastasis(ref).
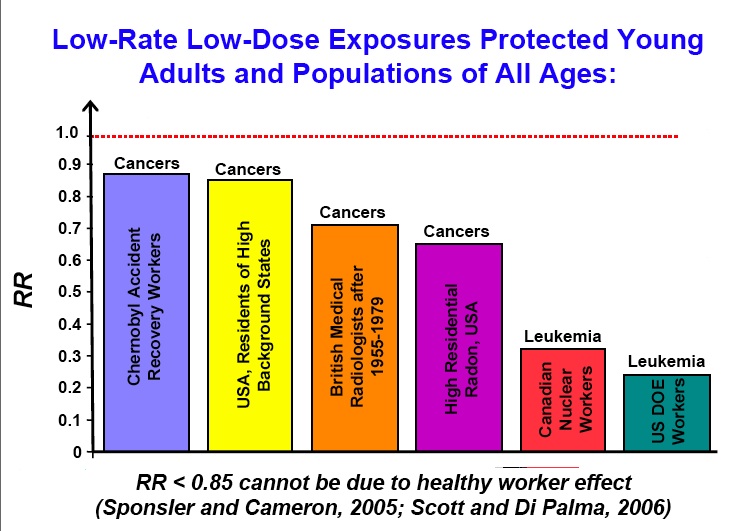
Epidemiological evidence of various kinds exists for the radiation hormesis hypothesis. For example, studies indicate that mortality of populations varies inversely with altitude and amount of natural background radiation.
The June 2012 publication Cancer mortality, state mean elevations, and other selected predictorsreports: “This ecological inquiry compares cancer mortality rates in the U.S. to the predictor of natural background radiation (via land elevation means) along with eight other predictors thought to be associated with cancer mortality. Age-adjusted cancer mortality in 2006 was compared to the predictors of mean land elevation, percent of smokers, educational attainment, percent of population without health insurance, income, obesity, health perception, physical activity, and diet. Among the six predictors considered appropriate for multiple linear regression, three were found to be statistically significant; from strongest to weakest, these three were: smoking, land elevation, and educational attainment. The predictors of smoking and educational attainment have long been considered associated with cancer mortality. The finding that the predictor of land elevation / natural background radiation is inversely related to cancer mortality is another piece of evidence supporting the theory of radiationhormesis. In this study, land elevation / natural background radiation ranked second in predictive strength regarding cancer mortality, behind smoking and ahead of educational attainment. Since this is an ecological inquiry, no causal inferences can be made.”
Other epidemiological studies related to radiation hormesis have looked at cancer risk. For example, the December 2010 review publication [Cancer incidence and mortality after low-dose radiation exposure: epidemiological aspects]reports:“Current recommendations for limiting exposure to ionizing radiation are based on the linear-no-threshold (LNT) model for radiation carcinogenesis under which every dose, no matter how low, carries with it some cancer risk. In this review, epidemiological evidences are discussed that the LNT hypothesis is incorrect at low doses. A large set of data was accumulated that showed that cancer risk after ordinarily encountered radiation exposure (natural background radiation, medical X-rays, etc.) is much lower than projections based on the LNT model. The discovery of the low-level radiation hormesis (stimulating effect) implies a non-linear dose-response curve in the low-dose region. The further studies in this field will provide new insights about the mechanisms of radiation carcinogenesis.”
An epidemiological study looking at radon gas exposure risk also points to the presence of radiation hormesis. The December 2010 publication Epidemiological Evidence for Possible RadiationHormesis from Radon Exposure: A Case-Control Study Conducted in Worcester, MA reported: “Data from a case-control study of lung cancer and residential radon exposure conducted in Worcester County, Massachusetts, are presented. Lung cancer risk was estimated using conditional logistic regression models that controlled for demographic, smoking, and occupational exposure covariates. Preliminary exploratory analyses using lowess smoothing revealed a non-linear association between exposure and the log odds of lung cancer. Radon exposure was considered by using linear spline terms in order to model this nonlinearity. The best fit of this linear spline model to these data predicted a shift from a positive to a negative slope in the log-odds of lung cancer at a radon concentration of 70 Bq m(-3). A statistically significant decrease in cancer risk with increased exposure was found for values ≤ 157 Bq m(-3) normalized to the reference exposure of 4.4 Bq m(-3), the lowest radon concentration measured(adjusted odds ratio (AOR) [95% CI] = 0.42 [0.180, 1.00], p = 0.049). This result is consistent with those reported elsewhere that considered radon exposure with cubic spline terms (Thompson, RE et al. 2008). Furthermore, this model predicts an AOR that is numerically less than 1.0 for radon exposures up to 545 Bq m(-3) versus the above baseline, reference exposure.”
Graphic depiction of the stochastic hormetic relative risk model as given in Scott et al. (2009).
Biological experiments also establish the existence of radiation hormesis.
For example, the January 2012 publication Low-dose-rate, low-dose irradiation delays neurodegeneration in a model of retinitis pigmentosa reports: “The existence of radiation hormesis is controversial. Several stimulatory effects of low-dose (LD) radiation have been reported to date; however, the effects on neural tissue or neurodegeneration remain unknown. Here, we show that LD radiation has a neuroprotective effect in mouse models of retinitis pigmentosa, a hereditary, progressive neurodegenerative disease that leads to blindness. Various LD radiation doses were administered to the eyes in a retinal degeneration mouse model, and their pathological and physiological effects were analyzed. LD gamma radiation in a low-dose-rate (LDR) condition rescues photoreceptor cell apoptosis both morphologically and functionally. The greatest effect was observed in a condition using 650 mGy irradiation and a 26 mGy/minute dose rate. Multiple rounds of irradiation strengthened this neuroprotective effect. A characteristic up-regulation (563%) of antioxidative gene peroxiredoxin-2 (Prdx2) in the LDR-LD-irradiated retina was observed compared to the sham-treated control retina. Silencing the Prdx2 using small-interfering RNA administration reduced the LDR-LD rescue effect on the photoreceptors. Our results demonstrate for the first time that LDR-LD irradiation has a biological effect in neural cells of living animals. The results support that radiation exhibits hormesis, and this effect may be applied as a novel therapeutic concept for retinitis pigmentosa and for other progressive neurodegenerative diseases regardless of the mechanism of degeneration involved.”
Another study showing a radiation hormesis effect on the cell level is reported in the 2011 publication The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells: “Hormesis induced by low-dose ionizing radiation (LDIR) is often mirrored by its stimulation of cell proliferation. The mitogen-activated protein kinases (MAPK)/ extracellular-signal- regulated kinases (ERK) pathway is known to play important roles in cell growth. Therefore, this study was to examine the effects of LDIR on rat mesenchymal stem cell (MSC) proliferation and MAPK/ERK signaling pathway. Rat MSCs were isolated from the bone marrow from 6 to 8-week-old male Wistar rats and cultured in vitro. Exponentially growing cells within 4-5 passages were irradiated with low doses of X-rays at 20, 50, 75 and 100 mGy with a dose rate of 100 mGy/min. Cell proliferation was evaluated by counting total viable cell number with trypan-blue staining and MTT assay. Cell cycle changes were also evaluated by flow cytometry and the activation of MAPK/ERK signaling pathway was assayed by Western blotting. Results showed that LDIR at 50 and 75 mGy significantly stimulated the proliferation of rat MSCs with the most stimulating effect at 75 mGy. There was a significant increase in the proportion of S phase cells in MSCs in response to 75 mGy X-rays. Activation of several members in the MAPK/ERK signaling pathway, including c-Raf, MEK and ERK were observed in the cells exposed to 75 mGy X-rays. To define the role of ERK activation in LDIR-stimulated cell proliferation, LDIR-treated MSCs were pre-incubated with MEK specific inhibitor U0126, which completely abolished LDIR-increased phosphorylation of ERK and cell proliferation. These results suggest that LDIR stimulates MSC proliferations in the in vitro condition via the activation of MAPK/ERK pathway.”
Of course, there are fruit fly studies showing radiation hormesis. The 2009 publication [Low-dose rate irradiation induced hormesis, hypersensitivity and adaptive response in Drosophila melanogaster of radiosensitive strains] reports: “We have studied the adaptive response after chronic low dose irradiation (2.5 mGy/h) in wild type Drosophila melanogaster strains (Canton-S and Oregon-R), as well as mutant strains on DNA damage sensing (mei-41), DNA repair (mus209, mus210, mus309, rad54) and free radicals detoxification (sod). The effects of irradiation on the prolongation of the larval stage, pupa lethality, and imago whole body weight have been analyzed. The high dose irradiation (30 Gy, 0.05 Gy/s) induced prolongation of the prepupal period and lethality in all wild type and mutant strains under investigation. The chronic low dose irradiation resulted in shortening of the larval development period (hormetic effect) was observed in wild type (Oregon-R) and mutant (mus209) strains (absorbed dose was 20 cGy) of Drosophila. At the same time these strains demonstrated the hormetic effect after chronic low dose irradiation. The hypersensitivity effect was found in sod and rad54 larvae (20 cGy, prolongation of the prepural period), and rad54 and mei-41 pupa (40 cGy, increase of death rate). The larvae of hypersensitive strains and pupa of all strains under investigation did not have the adaptive response. The chronic irradiation in 6 and 60 cGy with the dose rate of 0.25 and 2.5 mGy/h induced the hormetic effect (imago whole body weight enhancement) Canton-S. The obtained results suggest the important role of free radical detoxification, of DNA damage sensing, and of DNA repair mechanisms in the whole organism radiation induced effects. The appearance of the adaptive response depends on the investigated effect and developmental stage of fly.”
The 2008 publication Evidence for radiation hormesis after in vitro exposure of human lymphocytes to low doses of ionizing radiation reports another of several cell-level studies. “Previous research has demonstrated that adding a very small gamma-ray dose to a small alpha radiation dose can completely suppress lung cancer induction by alpha radiation (a gamma-ray hormetic effect). Here we investigated the possibility of gamma-ray hormesis during low-dose neutron irradiation, since a small contribution to the total radiation dose from neutrons involves gamma rays. Using binucleated cells with micronuclei (micronucleated cells) among in vitro monoenergetic-neutron-irradiated human lymphocytes as a measure of residual damage, we investigated the influence of the small gamma-ray contribution to the dose on suppressing residual damage. We used residual damage data from previous experiments that involved neutrons with five different energies (0.22-, 0.44-, 1.5-, 5.9-, and 13.7-million electron volts [MeV]). Corresponding gamma-ray contributions to the dose were approximately 1%, 1%, 2%, 6%, and 6%, respectively. Total absorbed radiation doses were 0, 10, 50, and 100 mGy for each neutron source. We demonstrate for the first time a protective effect (reduced residual damage) of the small gamma-ray contribution to the neutron dose. Using similar data for exposure to gamma rays only, we also demonstrate a protective effect of 10 mGy (but not 50 or 100 mGy) related to reducing the frequency of micronucleated cells to below the spontaneous level.”
Hormesis effects have been observed in a small study of interventional radiologists.
The February 2012 publication Cellular adaptive response to chronic radiation exposure in interventional cardiologists reports: “Aims: Invasive cardiologists are the most exposed to ionizing radiation among health professionals and show an increased rate of somatic DNA damage. To evaluate the effects of chronic low-dose exposure to ionizing radiation on redox state and apoptotic activation. Methods and results: We enrolled 10 healthy exposed professionals (all interventional cardiologists, Group II, exposed: age = 38 ± 5 years) and 10 age- and gender-matched unexposed controls (Group I, non-exposed). Exposed subjects had a median exposure of 4 mSv/year (range 1-8) by film badge dosimetry (below lead apron). We measured reduced glutathione (GSH, a marker of antioxidant response) in erythrocytes and plasma generation of hydrogen peroxide (a marker of oxyradical stress) by ferrous oxidation-xylenol orange assay in plasma. In both groups, lymphocytes were isolated and caspase-3 activity (a marker of apoptotic response) measured at baseline and following 2 Gy in vitro irradiation. Exposed subjects showed a three-fold increase in hydrogen peroxide (Group I = 2.21 ± 1.03 vs. II = 6.51 ± 1.55 μM H(2)O(2) equivalents) and a 1.7-fold increase in GSH (I = 12.37 ± 1.22 vs. II = 20.61 ± 2.16 mM). Exposed subjects also showed higher values of caspase-3 activity, both at baseline and-more strikingly-following high-dose radiation challenge. Conclusion: In interventional cardiologists, chronic exposure to low-dose radiation is associated with an altered redox balance mirrored by an increase in hydrogen peroxide and with two possibly adaptive cellular responses: (i) an enhanced antioxidant defence (increase in GSH, counteracting increased oxyradical stress) and (ii) an increased susceptibility to apoptotic induction which might efficiently remove genetically damaged cells.”
After 30 years the relevance and even existence of radiation hormesis remains controversial. Some have argued that the statistical analyses establishing radiation hormesis are faulty. Other publications suggest that not enough is known about it to justify changing current radiation safety standards..
The 2012 publication A meta-analysis of evidence for hormesis in animal radiation carcinogenesis, including a discussion of potential pitfalls in statistical analyses to detect hormesis is a case in point: “ A database containing 800 datasets on the incidence of specific tumor types from 262 radiation carcinogenicity experiments identified in a comprehensive literature search through September 2000 was analyzed for evidence of hormesis. This database includes lifetime studies of tumorigenic responses in mice, rats, and dogs to exposures to alpha, beta, gamma, neutron, or x-ray radiation. A J-shaped dose response, in the form of a significant decreased response at some low dose followed by a significant increased response at a higher dose, was found in only four datasets from three experiments. Three of these datasets involved the same control animals and two also shared dosed animals; the J shape in the fourth dataset appeared to be the result of an outlier within an otherwise monotonic dose response. A meta-analysis was conducted to determine whether there was an excess of dose groups with decreases in tumor response below that in controls at doses below no-observed-effect levels (NOELs) in individual datasets. Because the probability of a decreased response is generally not equal to the probability of an increased response even in the null case, the meta-analysis focused on comparing the number of statistically significant diminished responses to the number expected, assuming no dose effect below the NOEL. Only 54 dose groups out of the total of 2579 in the database had doses below the dataset-specific NOEL and that satisfied an a priori criterion for sufficient power to detect a reduced response. Among these 54, a liberal criterion for defining a significant decreases identified 15 such decreases, versus 54 × 0.2 = 10.8 expected. The excess in significant reductions was accounted for almost entirely by the excess from neutron experiments (10 observed, 6.2 expected). Nine of these 10 dose groups involved only 2 distinct control groups, and 2 pairs from the 10 even shared dosed animals. Given this high degree of overlap, this small excess did not appear remarkable, although the overlap prevented a formal statistical analysis. A comprehensive post hoc evaluation using a range of NOEL definitions and alternative ways of restricting the data entering the analysis did not produce materially different results. A second meta-analysis found that, in every possible low dose range ([0, d] for every dose, d) of each of the radiation types, the number of dose groups with significantly increased tumorigenic responses was either close to or exceeded the number showing significantly reduced responses. This meta-analysis was considered to be the more definitive one. Not only did it take dose into account by looking for consistent evidence of hormesis throughout defined low-dose ranges, it was also potentially less susceptible to limitations in experimental protocols that would cause individual animals to respond in a non-independent fashion. Overall, this study found little evidence in a comprehensive animal radiation database to support the hormesis hypothesis. However, the ability of the database to detect a hormetic effect was limited both by the small number of dose groups with doses below the range where positive effects have been found in epidemiological studies (≤ 0.1 Gy) and by the limited power of many of these dose groups for detecting a decrease in response.”
Another example, the June 2012 publication Radiationhormesis: Autophagy and other cellular mechanisms reports: “Purpose: To review the cellular mechanisms of hormetic effects induced by low dose and low dose rate ionising radiation in model systems, and to call attention to the possible role of autophagy in some hormetic effects. Results and conclusions: Very low radiation doses stimulate cell proliferation by changing the equilibrium between the phosphorylated and dephosphorylated forms of growth factor receptors. Radioadaptation is induced by various weak stress stimuli and depends on signalling events that ultimately decrease the molecular damage expression at the cellular level upon subsequent exposure to a moderate radiation dose. Ageing and cancer result from oxidative damage under oxidative stress conditions; nevertheless, ROS are also prominent inducers of autophagy, a cellular process that has been shown to be related both to ageing retardation and cancer prevention. A balance between the signalling functions and damaging effects of ROS seems to be the most important factor that decides the fate of the mammalian cell when under oxidative stress conditions, after exposure to ionising radiation. Not enough is yet known on the pre-requirements for maintaining such a balance. Given the present stage of investigation into radiationhormesis, the application of the conclusions from experiments on model systems to the radiation protection regulations would not be justified.”
There has been a Growing understanding of the epigenetic mechanisms through which radiation hormesis works.
An important contribution in this regard was the 2009 publication Radiation-Stimulated Epigenetic Reprogramming of Adaptive-Response Genes in the Lung: An Evolutionary Gift for Mounting Adaptive Protection Against Lung Cancer. It establishes, among other things, that the hormetic protective effects of low-level gamma ray exposure can protect against lung cancers induced by alpha-ray exposure. “Humans are continuously exposed to low-level ionizing radiation from natural sources. However, harsher radiation environments persisted during our planet’s early years and mammals survived via an evolutionary gift – a system of radiation-induced natural protective measures (adaptive protection). This system includes antioxidants, DNA repair, apoptosis of severely damaged cells, epigenetically regulated apoptosis (epiapoptosis) pathways that selectively remove precancerous and other aberrant cells, and immunity against cancer. We propose a novel model in which the protective system is regulated at least in part via radiation-stress-stimulated epigenetic reprogramming (epireprogramming) of adaptive-response genes. High-dose radiation can promote epigenetically silencing of adaptive-response genes (episilencing), for example via promoter-associated DNA and/or histone methylation and/or histone deacetylation. Evidence is provided for low linear-energy-transfer (LET) radiation-activated natural protection (ANP) against high-LET alpha-radiation-induced lung cancer in plutonium-239 exposed rats and radon-progeny-exposed humans. Using a revised hormetic relative risk model for cancer induction that accounts for both epigenetic activation (epiactivation) and episilencing of genes, we demonstrate that, on average, >80% of alpha-radiation-induced rat lung cancers were prevented by chronic, low-rate gamma-ray ANP. Interestingly, lifetime exposure to residential radon at the Environmental Protection Agency’s action level of 4 pCi L−1 appears to be associated with on average a > 60% reduction in lung cancer cases, rather than an increase. We have used underlined italics to indicate newly introduced terminology.”
The single most-important biological pathway responsible for radiation hormesis is probably the activation of Nrf2 by radiation-created oxidative stress.
This observation became clear to me as an “of course” matter as soon as I begin digging into the area of radiation hormesis. I have written extensively about Nrf2 in this blog. Nrf2 is the master regulator of the body’s response to stresses of most kinds. Activating hundreds of the body’s natural antioxidant and stress defense genes, the Nrf2/Keap1 pathway provides a central mechanism for most if not all forms of hormesis. Specifically, you can see the blog entries The pivotal role of Nrf2. Part 1 – a new view on the control of oxidative damage and generation of hormetic effects, The pivotal role of Nrf2. Part 2 – foods, phyto-substances and other substances that turn on Nrf2 and The pivotal role of Nrf2. Part 3– Is promotion of Nrf2 expression a viable strategy for human human healthspan and lifespan extension?.
Lending credence to this perception is the December 2011 publication Ionizing radiation activates the Nrf2 antioxidant response. “The transcription factor NF-E2-related factor 2 (Nrf2) binds the antioxidant DNA response element (ARE) to activate important cellular cytoprotective defense systems. Recently several types of cancers have been shown to overexpress Nrf2, but its role in the cellular response to radiation therapy has yet to be fully determined. In this study, we report that single doses of ionizing radiation from 2 to 8 Gy activate ARE-dependent transcription in breast cancer cells in a dose-dependent manner, but only after a delay of five days. Clinically relevant daily dose fractions of radiation also increased ARE-dependent transcription, but again only after five days. Downstream activation of Nrf2-ARE-dependent gene and protein markers, such as heme oxygenase-1, occurred, whereas Nrf2-deficient fibroblasts were incapable of these responses. Compared with wild-type fibroblasts, Nrf2-deficient fibroblasts had relatively high basal levels of reactive oxygen species that increased greatly five days after radiation exposure. Further, in vitro clonogenic survival assays and in vivo sublethal whole body irradiation tests showed that Nrf2 deletion increased radiation sensitivity, whereas Nrf2-inducing drugs did not increase radioresistance. Our results indicate that the Nrf2-ARE pathway is important to maintain resistance to irradiation, but that it operates as a second-tier antioxidant adaptive response system activated by radiation only under specific circumstances, including those that may be highly relevant to tumor response during standard clinical dose-fractionated radiation therapy.”
As a matter if fact, radiation oncologists have been mainly concerned with Nrf2 and its hormetic effects because they could reduce the traditional cancer cell killing impacts of radiation cancer therapies. Another relevant publication in this regard is the 2010 report Relationship between Radiosensitivity and Nrf2 Target Gene Expression in Human Hematopoietic Stem Cells. “NFE2-related factor 2 (Nrf2), which belongs to the cap “n” collar family of basic region leucine zipper transcription factors, is a key protein in the coordinated transcriptional induction of expression of various antioxidant genes. The purpose of this study was to analyze the expression of Nrf2 target genes, such as heme oxygenase 1 (HO-1), ferritin heavy polypeptide 1 (FTH1), NAD(P)H dehydrogenase, quinone 1 (NQO1), glutamate-cysteine ligase catalytic subunit, glutamate-cysteine ligase modifier subunit, glutathione reductase (GSR) and thioredoxin reductase 1 (TXNRD1), after X irradiation of CD34+ cells that were prepared from human placental/umbilical cord blood hematopoietic stem cells (HSCs). We evaluated the relationship between radiosensitivity and expression of Nrf2 target genes in HSCs. The number of colony-forming cells derived from 2-Gy-irradiated HSCs decreased to approximately 20% of the nonirradiated control. At the same time, the mRNA expression of HO-1, FTH1, NQO1, GSR and TXNRD1 was significantly increased after X irradiation. A statistically significant negative correlation was observed between the surviving fraction of HSCs and the intrinsic NQO1 mRNA expression, indicating that HSCs in which NQO1 mRNA levels are low may also be radioresistant. The present results suggest that the antioxidant system associated with Nrf2 is involved in the radiosensitivity of HSCs.”
It is interesting though that in the publication of those directly concerned with radiation hormesis, I have yet to come across a mention of Nrf2.
Radiation hormesis is still not a mainline view
There is epidemiological, experimental and genetic pathway evidence for the existence of radiation hormesis. However, given political and social situations, the radiation safety establishment has chosen to view even low-level radiation as unsafe, despite a growing body of scientific evidence contrary to this view.
This situation is characterized in the April 2012 publication Hormesis: A peep in to the human nature. “Hormesis is a term to denote biphasic dose response to an agent which reveals a stimulatory or beneficial effects at low dose and an inhibitory or toxic consequence at a higher dose or concentration. Hormesis is a concept which is involved to biphasic dose response effects of environmental agents including ionizing radiation. Proponents of anti-nuclear lobby brand the idea of hormesis as the privilege of the cranks, and the ill informed. UNSECAR in 1958 and 1 CRP in 1959 adopted linear non threshold theory (LNT) in contrast to hormetic or biphasic relation. These organizations based their recommendation by extrapolation of the studies done on atomic bomb survivors who had received higher doses of radiation. According to LNT theory (a) The effect of low doses or radiation can be estimated by linear extrapolation from effects observed by high doses, and (b) There are not any safe doses as even very low doses of ionizing radiation produce some biological effect (Mortazan). — Science dislikes any vacuum of ideas, experiments, and evidence. — It is generally assumed that science is based on specific results which is independent of personalities; in other words, the interpretation is secular. However, in reality, interpretation of results may to an extent, depend on the position of the interpreter. The frenzied debates about hormesis in radiation or global warming attest to the above assertion. Jamea Miller observed mutation following radiation of Drosophila. He assumed a linear relation between radiation and mutation though his experiments did not include very low doses of radiation. Catastrophe following nuclear war on Hiroshima and Nagasaki has resulted in radiophobia and an aversion to the concept of hormesis. The evidence for hormesis is accumulating. According to UNSECAR (1994), amongst A-bomb survivors from Hiroshima and Nagasaki, who received doses below 200 mSv, there was no incidence of cancer deaths. And deaths due to leukemia in the sub group who received less than 100 mSv was less than age matched controls. Nambi and Soman in 1987 have demonstrated a significantly reduced death due to cancer in high back ground areas versus areas of low background radiation in Kerala. In a Canadian survey the mortality caused by cancer at nuclear plants was 58% lower than national average. (Abbat 1983). Thus, there is no evidence of harmful effects of radiation at lower doses. Yet, most people tend to go by LNT model including regulatory authorities. — There are instances in the history of medicine where intuitive decision have been disastrous. It took a while to establish a link between administration of saturated oxygen and retrolenticular fibroblasia in neonates. It was felt that life sustaining oxygen can never harm human life even at higher concentration. Thus, establishing a casual link between retrolenticular hyperplasia and saturated oxygen took considerable time by which time innumerable neonates were affected. The biphasic action of chlorpromazine as protector and senstiser at various concentrations is yet another instance of concentration dependent behavior. There are innumerable such examples of biphasic phenomenon. — Hormesis is integral to the normal physiological function of cells and organisms (Mark Mattson) Adaptive responses evolve due to biphasic response to many physical and chemical stimuli. Hormesis also has played a crucial role in the evolution of life. Life had to evolve despite the harsh environmental conditions, including higher cosmic radiation that is seen now. Cellular mechanism including signaling pathways responsible for adaptive pathways are emerging. Now, there is a mechanistic explanation for a possible hermetic response for a wide range of stimulants, including that for ionizing radiation. — Radiophobia has propelled many a nuclear activitists and scientists in to a narrow tunnel vision, obscuring the evidence, as well as disabling a congent thinking. Men are no Drosophila or cells in the Petri dish . Complex biological systems behave much differently than most people concede. Failure to acknowledge hormesis is also a failure to realize essential realities of biological complexity.”
There is a slow broadening of views in the area of radiobiology to where hormesis is being accepted.
The June 2012 publication Changing paradigms in radiobiologysummarizes the nature of this shift: “The last 25 years have seen a major shift in emphasis in the field of radiobiology from a DNA-centric view of how radiation damage occurs to a much more biological view that appreciates the importance of macro-and micro-environments, hierarchical organization, underlying genetics, evolution, adaptation and signaling at all levels from atoms to ecosystems. The new view incorporates concepts of hormesis, nonlinear systems, bioenergy field theory, uncertainty and homeodynamics. While the mechanisms underlying these effects and responses are still far from clear, it is very apparent that their implications are much wider than the field of radiobiology. This reflection discusses the changing views and considers how they are influencing thought in environmental and medical science and systems biology.”
For years, voices have been raised calling for acknowledgement of the existence of radiation hormesis and for revision of the LNT (linear no-threshold) standards for radiation protection which state that radiation exposure at any dose is biologically damaging. These calls have not gotten anywhere so far.
The 2008 publication It’s time for a new low-dose-radiation risk assessment paradigm–one that acknowledges hormesis is one of many over the years calling for revision of the LNT. “The current system of radiation protection for humans is based on the linear-no-threshold (LNT) risk-assessment paradigm.  Perceived harm to irradiated nuclear workers and the public is mainly reflected through calculated hypothetical increased cancers. The LNT-based system of protection employs easy-to-implement measures of radiation exposure. Such measures include the equivalent dose (a biological-damage-potential-weighted measure) and the effective dose (equivalent dose multiplied by a tissue-specific relative sensitivity factor for stochastic effects). These weighted doses have special units such as the sievert (Sv) and millisievert (mSv, one thousandth of a sievert). Radiation-induced harm is controlled via enforcing exposure limits expressed as effective dose. Expected cancer cases can be easily computed based on the summed effective dose (person-sievert) for an irradiated group or population. Yet the current system of radiation protection needs revision because radiation-induced natural protection (hormesis) has been neglected. A novel, nonlinear, hormetic relative risk model for radiation-induced cancers is discussed in the context of establishing new radiation exposure limits for nuclear workers and the public.”
Perceived harm to irradiated nuclear workers and the public is mainly reflected through calculated hypothetical increased cancers. The LNT-based system of protection employs easy-to-implement measures of radiation exposure. Such measures include the equivalent dose (a biological-damage-potential-weighted measure) and the effective dose (equivalent dose multiplied by a tissue-specific relative sensitivity factor for stochastic effects). These weighted doses have special units such as the sievert (Sv) and millisievert (mSv, one thousandth of a sievert). Radiation-induced harm is controlled via enforcing exposure limits expressed as effective dose. Expected cancer cases can be easily computed based on the summed effective dose (person-sievert) for an irradiated group or population. Yet the current system of radiation protection needs revision because radiation-induced natural protection (hormesis) has been neglected. A novel, nonlinear, hormetic relative risk model for radiation-induced cancers is discussed in the context of establishing new radiation exposure limits for nuclear workers and the public.”
Given that individuals may vary significantly in their hormetic responses to radiation, establishing standards to replace LNT could be a daunting task.
Wrapping it up
It is unfortunate that radiation safety standards and therefore radiation hormesis seem to be so bound up with nuclear energy safety issues, issues of great public concern where most people want to be on the safe side. It is thought by some that radiation hormesis is mainly an argument put forward by the nuclear industry to lessen the regulatory burden on it, and this may to some extent be so. However, science is science.
My personal opinion is that radiation hormesis is real and important, first because of the collective research evidence to this effect, second because it is very well established that controlled oxidative stress induces hormesis, so it can be expected that free radicals produced by radiation would produce such stress and induce hormesis, and third, because the key biological pathway, the Nrf2/Keap1 pathway, is now understood.
Radiation hormesis is ancient and evolutionary but good news, providing us with layers of protection we did not know about.
It also seems clear to me that the LNT standards for radiation safety should be revised given the presence of radiation hormesis, but how much so will require further concerted study and that in turn will require a new public safety initiative. Fully acknowledging the effects of radiation hormesis conceivably could significantly reduce public concern for radiation safety from x-rays and radon as well as nuclear power plants.



I strongly agree that the scientific evidence for a hormetic effect of low dose radiation cannot be denied and must no longer be ignored. There is even a pre-natal, intrauterine beneficial effect of low dose radiation. Randy Jirtle from Duke University presented his most recent research at the NIH Wednesday afternoon lecture series and clearly showed in the variable-yellow Agouti mice model (metastable epiallele gene), pre-natal exposure of 0.5 cGy to 3 cGy. Above this threshold, the effects of radiation are deleterious. Keep in mind that Randy Jirtle’s model is an epigenetic model of DNA methylation that is phenotypically manifest by brown coat color and normal weight. When DNA methylation was incomplete, the mice would have a yellow coat color and would be obese. These low dose prenatal exposures to XRT resulted in hypermethylation of the Agouti locus resulting in brown coat color and no obesity of the offspring. The coat color distribution and methylation status of the Agouti locus was “dose dependent”.
Even more interesting was that the administration of anti-oxidants extinguished (mitigated) this effect of low dose XRT on DNA methylation. This suggests that the mechanism on how XRT affects epigenetics is via free radicals. This is very compelling evidence for the free radical mediated epigenetic alternation of gene methylation as the hormetic explanation of low dose XRT. The other interesting (unexplained) thing is that this phenomena was only seen in male mice fetuses, NOT in females.
You can view his lecture on Youtube at the following link:
http://www.youtube.com/watch?v=lcaQWSejufI
The part on the hormetic effect of XRT is in the 2nd half of the lecture
James watson
Thank you for your important update to this blog entry. Yes, I completely agree. I think that some of the key words in the near future will be hormesis, epigenetics, and DNA methylation – along with phytochemicals and non-coding RNAs. I looked at Randy Jirtle’s YouTube presentation and fell very much in love with it. Perhaps it is that we both got our starts in computer science. I like his analogy that genetics provides the hardware and epigenetics provides the software. And that we are still in the hardware stage, like 1956 in the computer revolution. I strongly recommend watching that presentation to my readers. Again, it is at http://www.youtube.com/watch?v=lcaQWSejufI
Vince
There are several “natural experiments” that could be done to prove/disprove the beneficial effects of low dose radiation. Randy Jirtle suggested the first one, below. I have suggested another one to follow.
1. Following the offspring (there were 6,000 of them) of pregnant females who were exposed to 0.5 to 100 cGy of XRT from the atomic bomb blast zones of Nagasaki and Hiroshima. A CpG methylation status of metastable epialleles in these offspring would be easy to do. (Ex: regulatory elements of imprinted genes)
2. Following individuals who expose themselves to radon gas in the US (4 old mines near the towns of Boulder and Basin in Montana) and Europe (Gasteiner Heilstollen, Austria; Schlema & Sybyllenbad, Germany; Radium Palace, Czech Republic) for health effects. Some of these individuals have been doing this on a regular basis for 10-20 years. A study of NR2F/Keap pathway gene expression in a leukocyte microarray transcriptome post exposure would be of great interest. Here is an article on this: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2477705/
Pingback: Mitohormesis | AGING SCIENCES – Anti-Aging Firewalls
Pingback: Editorial -Bridging the Great Divide | AGING SCIENCES – Anti-Aging Firewalls