By Vince Giuliano and James P Watson
Continuing a tradition in this blog of reviewing particularly interesting phytochemicals, we here discuss research on health-related properties and applications of anacardic acid. Although much of the research on anacardic acid has been conducted over a 40 year period, we have not reported on it previously in this blog. Its epigenetic and other properties applicable to medicine are unique and are of high current interest. And there have been important new findings about it in just the last two years.
Basics about anacardic acid
The medicinal use of anacardic acid goes back long before it was called that. “The traditional Ayurveda depicts nutshell oil as a medicinal remedy for alexeritic, amebicidal, gingivitis, malaria and syphilitic ulcers. However, the enduring research and emerging evidence suggests that AA (anacardic acid) could be a potent target molecule with bactericide, fungicide, insecticide, anti-termite and molluscicide properties and as a therapeutic agent in the treatment of the most serious pathophysiological disorders like cancer, oxidative damage, inflammation and obesity. Furthermore, AA was found to be a common inhibitor of several clinically targeted enzymes such as NFκB kinase, histone acetyltransferase (HATs), lipoxygenase (LOX-1), xanthine oxidase, tyrosinase and ureases(ref).”
Modern history of the substance goes back to a publication in 1847 which described an acid found in the shells of cashew nuts named anacardic acid. Later, the substance was found to consist of a family of related acid compounds(ref). It was used for many years for industrial purposes. “Anacardic acid is the main component of cashew nutshell liquid (CNSL), and finds use in the chemical industry for the production of cardanol, which is used for resins, coatings, and frictional materials. Cardanol is used to make phenalkamines, which are used as curing agents for the durable epoxy coatings used on concrete floors[9](ref). Life sciences research interest in anacardic acid it has been accelerating with the rise in knowledge about molecular biology and epigenetics. Once-unknown mechanisms underlying its antibacterial, anti-tumor, anti-inflammatory and other interesting properties are increasingly better understood, and the acid is of increasing interest for its practical anti-cancer and other health-inducing properties.
Anacardic acids occurs naturally in cashew nuts (the most accessible natural dietary source), mangos, and cashew apples. It is also found in Gingko biloba(ref), and geraniums.

 Image source Cashew nut in shell on a cashew apple
Image source Cashew nut in shell on a cashew apple
Image source Raw cashew nuts in shells and cashew apples
Image source Raw cashew nut in split shell
The cashew nut developes in a shell which grows at the end of the cashew apple. Anacardic acid is mainly found in the shell.
Anacardic acid is a collective name. Chemists have identified dozens of molecular variants, The basic structure is this, where the tail element R is variable depending on the particular acid concerned: 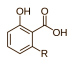
A variant among those appearing in cashew nuts is : 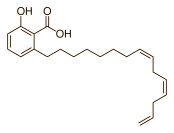
See Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. In this blog entry we will use the singular anacardic acid except where there is a need to discuss individual acid components.
Summing much of it up: “Anacardic acid, isolated from cashew shells or several other medicinal plants, is the general name given to a family of four different 6-alkyl salicyclic acids having varying degrees of unsaturation in the 15-carbon alkyl chain.1 These compounds are associated with anti-inflammatory, anti-tumor, molluscicidal, and anti-microbial activity. Literature frequently sites and gives the name anacardic acid to the completely-saturated compound (6-pentadecyl salicylic acid). Anacardic acid inhibits the histone acetyltransferase (HAT) activity of the transcription co-activators p300 and p300/CREB-binding protein-associated factor (pCAF) with IC50 values of 8.5 and 5 µM, respectively.2 At 25 µmol/L, anacardic acid suppresses NF-κB activation, inhibits IκB-α phosphorylation, and prohibits p65 nuclear translocation in KBM-5 cells.3(ref)”
“Diverse biological activities for the AAs have been described, including antimicrobial activity against methicillin-resistant bacteria [20–22], gastroprotection [23], and inhibition of the activity of several clinically targeted enzymes, such as lipoxygenase [24, 25], cyclooxygenase [26, 27], and histone acetyltransferases [28, 29]. It has been also demonstrated that AAs modulate the NF- B signaling pathway and inhibit tumor angiogenesis indicating that these compounds could be a therapeutic option in preventing or treating cancer [30–32](ref).”
Biological and health-related actions of anacardic acid
Like glucosamine and trehalose which we recently covered, there are many different molecular mechanisms and effects exercised by anacardic acids. Most interesting among many effects are the anti-inflammatory effects, the epigenetic effects and the antibacterial effects
Anti-inflammatory effects
Anacardic acid is an inhibitor of inflammation re suppression of expression of NF-kappaB. This is the start of the story – Anacardic acid can down regulate inflammation by inhibiting both the inducible and constitutive NF-kB. It also can reduce inflammation by inhibiting the lipoxygenases. This is probably due to HAT inhibition. Thus anacardic acid is a “epigenetic modulator of inflammation” by inhibiting the activation/expression of genes normally “turned on” by NF-kB. As you may know, TNF-alpha, IL-1beta, Lipopolysaccharide (LPS), PMA, OA, and EGF are main molecular activators of NF-kB. Anacardic acid inhibited the activation of NF-kB by all of these triggers of inflammation. This is why it appears to be an “upstream” inhibitor of NF-kB. Anacardic acid inhibited NF-kB induced gene expression in a dose-dependent relationship in vitro, with doses as low as 25 micro mol/L
The 2005 publication Lipoxygenase Inhibitory Activity of Anacardic Acidsreported: “6[8‘(Z)-Pentadecenyl]salicylic acid, otherwise known as anacardic acid (C15:1), inhibited the linoleic acid peroxidation catalyzed by soybean lipoxygenase-1 (EC 1.13.11.12, type 1) with an IC50 of 6.8 μM. The inhibition of the enzyme by anacardic acid (C15:1) is a slow and reversible reaction without residual activity. The inhibition kinetics analyzed by Dixon plots indicates that anacardic acid (C15:1) is a competitive inhibitor and the inhibition constant, KI, was obtained as 2.8 μM. Although anacardic acid (C15:1) inhibited the linoleic acid peroxidation without being oxidized, 6[8‘(Z),11‘(Z)-pentadecadienyl]salicylic acid, otherwise known as anacardic acid (C15:2), was dioxygenated at low concentrations as a substrate. In addition, anacardic acid (C15:2) was also found to exhibit time-dependent inhibition of lipoxygenase-1. The alk(en)yl side chain of anacardic acids is essential to elicit the inhibitory activity. However, the hydrophobic interaction alone is not enough because cardanol (C15:1), which possesses the same side chain as anacardic acid (C15:1), acted neither as a substrate nor as an inhibitor.”
The 2008 publication Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosisreported: “Anacardic acid (6-pentadecylsalicylic acid) is derived from traditional medicinal plants, such as cashew nuts, and has been linked to anticancer, anti-inflammatory, and radiosensitization activities through a mechanism that is not yet fully understood. Because of the role of nuclear factor-kappaB (NF-kappaB) activation in these cellular responses, we postulated that anacardic acid might interfere with this pathway. We found that this salicylic acid potentiated the apoptosis induced by cytokine and chemotherapeutic agents, which correlated with the down-regulation of various gene products that mediate proliferation (cyclin D1 and cyclooxygenase-2), survival (Bcl-2, Bcl-xL, cFLIP, cIAP-1, and survivin), invasion (matrix metalloproteinase-9 and intercellular adhesion molecule-1), and angiogenesis (vascular endothelial growth factor), all known to be regulated by the NF-kappaB. We found that anacardic acid inhibited both inducible and constitutive NF-kappaB activation; suppressed the activation of IkappaBalpha kinase that led to abrogation of phosphorylation and degradation of IkappaBalpha; inhibited acetylation and nuclear translocation of p65; and suppressed NF-kappaB-dependent reporter gene expression. Down-regulation of the p300 histone acetyltransferase gene by RNA interference abrogated the effect of anacardic acid on NF-kappaB suppression, suggesting the critical role of this enzyme. Overall, our results demonstrate a novel role for anacardic acid in potentially preventing or treating cancer through modulation of NF-kappaB signaling pathway.”
Epigenetic effects
Anacardic acid is a HAT (Histone Acetyl Transferase) inhibitor, meaning that it can help keep key genes such as those involved in inflammation, turned off. In fact, it is often used in laboratory experiments for that purpose. Specifically, Anacardic acid inhibits the Tip60 HAT as well as the p300/CBP HAT. Inhibiting the HAT p300/CBP is thought to be a great strategy for cancer treatment. p300/CBP can acetylate all four core histone proteins of the nucleosome on their lysine amino acids located on the histone protein tails. Both anacardic acid and curcumin and chemical variants of these have been investigated for their p300 HAT inhibiting capabilities.
The 2003 publication Small Molecule Modulators of Histone Acetyltransferase p300 reported:: “Histone acetyltransferases (HATs) are a group of enzymes that play a significant role in the regulation of gene expression. These enzymes covalently modify the N-terminal lysine residues of histones by the addition of acetyl groups from acetyl-CoA. Dysfunction of these enzymes is often associated with the manifestation of several diseases, predominantly cancer. Here we report that anacardic acid from cashew nut shell liquid is a potent inhibitor of p300 and p300/CBP-associated factor histone acetyltranferase activities. Although it does not affect DNA transcription, HAT-dependent transcription from a chromatin template was strongly inhibited by anacardic acid. Furthermore, we describe the design and synthesis of an amide derivative N-(4-chloro-3-trifluoromethyl-phenyl)-2-ethoxy-6-pentadecyl-benzamide (CTPB) using anacardic acid as a synthon, which remarkably activates p300 HAT activity but not that of p300/CBP-associated factor. Although CTPB does not affect DNA transcription, it enhances the p300 HAT-dependent transcriptional activation from in vitro assembled chromatin template. However, it has no effect on histone deacetylase activity. These compounds would be useful as biological switching molecules for probing into the role of p300 in transcriptional studies and may also be useful as new chemical entities for the development of anticancer drugs.”
The 2013 publication Anacardic acid, a histone acetyltransferase inhibitor, modulates LPS-induced IL-8 expression in a human alveolar epithelial cell line A549 reports: “Objective and design: The histone acetylation processes, which are believed to play a critical role in the regulation of many inflammatory genes, are reversible and regulated by histone acetyltransferases (HATs), which promote acetylation, and histone deacetylases (HDACs), which promote deacetylation. We studied the effects of lipopolysaccharide (LPS) on histone acetylation and its role in the regulation of interleukin (IL)-8 expression. Material: A human alveolar epithelial cell line A549 was used in vitro. Methods: Histone H4 acetylation at the IL-8 promoter region was assessed by a chromatin immunoprecipitation (ChIP) assay. The expression and production of IL-8 were evaluated by quantitative polymerase chain reaction and specific immunoassay. Effects of a HDAC inhibitor, trichostatin A (TSA), and a HAT inhibitor, anacardic acid, were assessed. Results: Escherichia coli-derived LPS showed a dose- and time-dependent stimulatory effect on IL-8 protein production and mRNA expression in A549 cells in vitro. LPS showed a significant stimulatory effect on histone H4 acetylation at the IL-8 promoter region by ChIP assay. Pretreatment with TSA showed a dose-dependent stimulatory effect on IL-8 release from A549 cells as compared to LPS alone. Conversely, pretreatment with anacardic acid inhibited IL-8 production and expression in A549 cells. Conclusion: These data suggest that LPS-mediated proinflammatory responses in the lungs might be modulated via changing chromatin remodeling by HAT inhibition.”
Suppression of ROS-induced damage – Actions in mitochondria
Anacardic acids also are potent mitochondrial uncouplers. General anesthetics are also mitochondrial uncouplers which uncouple oxidative phosphorylation from the electron transport chain. This reduces ATP synthesis and also reduces ROS leak. Anacardic acid can uncouple oxidative phosphorylation from the electron transport chain. As a result, anacardic acid is a “mitochondrial-specific antioxidant” because they can change the membrane potential of the inner mitochondrial membrane, thereby reducing ROS leak.
Anacardic acid can exercise an uncoupling effect in liver mitochondria. The 2000 publication Uncoupling effect of anacardic acids from cashew nut shell oil on oxidative phosphorylation of rat liver mitochondriareports:“Anacardic acids are one of natural products found in not only the cashew nut shell oil but also the nut and fruit juice. The present study was conducted to investigate the uncoupling effect of anacardic acids on oxidative phosphorylation of rat liver mitochondria using succinate (plus rotenone) as a substrate. Four anacardic acids with C15:0, C15:1, C15:2 or C15:3 as an alkyl side chain exhibited uncoupling effects similar to the classical uncoupler, 2,4-dinitrophenol on ADP/O ratio, state 4 and respiratory control ratio (RCR). Anacardic acid with C15:1 side chain was most effective for uncoupling of these compounds. Salicylic acid, which has no alkyl side chain, exhibited a very weak uncoupling effect on oxidative phosphorylation. When the carboxyl group in anacardic acids was lost converting them to the corresponding cardanols, uncoupling activity dramatically decreased regardless of the number of double bonds in the long alkyl chain. These results suggest that the C15 alkyl side chain as well as the carboxyl group may play an important role in assisting the uncoupling activity of anacardic acids in liver mitochondria of animals. This study provides the first evidence of an uncoupling effect of anacardic acids on liver mitochondria.”
Anacardic acid can also help neutralize excess charge in liposomal membranes
The 2002 publication Anacardic acid-mediated changes in membrane potential and pH gradient across liposomal membranesreports: “We have previously shown that anacardic acid has an uncoupling effect on oxidative phosphorylation in rat liver mitochondria using succinate as a substrate (Life Sci. 66 (2000) 229–234). In the present study, for clarification of the physicochemical characteristics of anacardic acid, we used a cyanine dye (DiS-C3(5)) and 9-aminoacridine (9-AA) to determine changes of membrane potential (ΔΨ) and pH difference (ΔpH), respectively, in a liposome suspension in response to the addition of anacardic acid to the suspension. — These results provide the evidence for a unique function of anacardic acid, dissimilar to carbonylcyanide p-trifluoromethoxyphenylhydrazone or valinomycin, in that anacardic acid behaves as both an electrogenic (negative) charge carrier driven by ΔΨ, and a ‘proton carrier’ that dissipates the transmembrane proton gradient formed.” Assuming the same effect applies to mitochondrial membranes, then anacardic acid can help neutralize excess charge buildup and consequent ROS generation from old leaky mitochondria.
Regulating the DNA damage response in tumor cells
The2006 publication Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation: reports: “Histone acetyltransferases (HATs) regulate transcription, chromatin structure and DNA repair. Here, we utilized a novel HAT inhibitor, anacardic acid, to examine the role of HATs in the DNA damage response. Anacardic acid inhibits the Tip60 HAT in vitro, and blocks the Tip60-dependent activation of the ATM and DNA–PKcs protein kinases by DNA damage in vivo. Further, anacardic acid sensitizes human tumor cells to the cytotoxic effects of ionizing radiation. These results demonstrate a central role for HATs such as Tip60 in regulating the DNA damage response. HAT inhibitors provide a novel therapeutic approach for increasing the sensitivity of tumors to radiation therapy.”
Because anacardic acid is a powerful HAT Inhibitor, it has many potential applications in medicine not only for cancer but for several other pathological conditions as well.
The 2009 publication Histone acetyl transferases as emerging drug targets reports: “Post-translational modifications, such as acetylation or phosphorylation, play a crucial role in the regulation of gene transcription in eukaryotes. Different subtypes of histone acetyl transferases (HATs) catalyze the acetylation of histones on specific lysine residues. A potential role of HATs in the pathology of cancer, asthma, COPD and viral infection has been described. This indicates that specific HAT inhibitors are potential tools for pharmacological research and might find therapeutic applications. This review focuses on the role of the HATs p300, CBP, PCAF and GCN5 in different diseases and the development of small-molecule inhibitors of these enzymes as potential drugs.”
Anti-cancer effects
It has been known for a long time that anacardic acid can act as a anticancer compound. See the 1993 publication Antitumor agents from the cashew (Anacardium occidentale) apple juice. And it can radiosensitize a tumor to external beam XRT. Many labs have already started synthesizing synthetic analogs of anacardic acid and are now trying them on cancer. Some of these anacardic acid analogs are already in clinical trials(ref).
The 2003 publication Small Molecule Modulators of Histone Acetyltransferase p3reported: “Histone acetyltransferases (HATs) are a group of enzymes that play a significant role in the regulation of gene expression. These enzymes covalently modify the N-terminal lysine residues of histones by the addition of acetyl groups from acetyl-CoA. Dysfunction of these enzymes is often associated with the manifestation of several diseases, predominantly cancer. Here we report that anacardic acid from cashew nut shell liquid is a potent inhibitor of p300 and p300/CBP-associated factor histone acetyltranferase activities. Although it does not affect DNA transcription, HAT-dependent transcription from a chromatin template was strongly inhibited by anacardic acid. Furthermore, we describe the design and synthesis of an amide derivative N-(4-chloro-3-trifluoromethyl-phenyl)-2-ethoxy-6-pentadecyl-benzamide (CTPB) using anacardic acid as a synthon, which remarkably activates p300 HAT activity but not that of p300/CBP-associated factor. Although CTPB does not affect DNA transcription, it enhances the p300 HAT-dependent transcriptional activation from in vitro assembled chromatin template. However, it has no effect on histone deacetylase activity. These compounds would be useful as biological switching molecules for probing into the role of p300 in transcriptional studies and may also be useful as new chemical entities for the development of anticancer drugs.”
We note that curcumin appears to be unique among other phytosubstances besides anacardic acid in being an inhibitor of p300/CBP. See Curcumin is an Inhibitor of p300 Histone Acetylatransferase.
Synthesis of Benzamides Related to Anacardic Acid and Their Histone Acetyltransferase (HAT) Inhibitory Activities(2008) reports: “The subset of 4-cyano-3-trifluoromethylphenylbenzamides with shorter chains exhibited activities similar to that of AA, as they behaved as human p300 inhibitors, induced a decrease in histone acetylation levels in immortalized HEK cells, and counteracted the action of the HDAC inhibitor SAHA in MCF7 breast cancer cells.
The 2011 publication Anacardic acid inhibits estrogen receptor alpha-DNA binding and reduces target gene transcription and breast cancer cell proliferation discusses mechanicisms of action of anacardic acid in breast cancer cells beyond HAT inhibition, It relates: “Anacardic acid (2-hydroxy-6-alkylbenzoic acid) is a dietary and medicinal phytochemical with established anticancer activity in cell and animal models. The mechanisms by which anacardic acid inhibits cancer cell proliferation remain undefined. Anacardic acid 24:1ω5(AnAc 24:1ω5) was purified from geranium (Pelargonium × hortorum) and shown to inhibit the proliferation of estrogen receptor α (ERα)-positive MCF-7 and endocrine-resistant LCC9 and LY2 breast cancer cells with greater efficacy than ERα-negative primary human breast epithelial cells, MCF-10A normal breast epithelial cells, and MDA-MB-231 basal-like breast cancer cells. AnAc 24:1ω5 inhibited cell cycle progression and induced apoptosis in a cell-specific manner. AnAc 24:1ω5 inhibited estradiol (E2)-induced estrogen response element (ERE) reporter activity and transcription of the endogenous E2-target genes: pS2, cyclin D1, and cathepsin D in MCF-7 cells. AnAc 24:1ω5 did not compete with E2 for ERα or ERβ binding, nor did AnAc 24:1ω5 reduce ERα or ERβ steady state protein levels in MCF-7 cells; rather, AnAc 24:1ω5 inhibited ER-ERE binding in vitro. Virtual Screening with the molecular docking software Surflex evaluated AnAc 24:1ω5 interaction with ERα ligand binding and DNA binding domains (LBD and DBD) in conjunction with experimental validation. Molecular modeling revealed AnAc 24:1ω5 interaction with the ERα DBD but not the LBD. Chromatin immunoprecipitation (ChIP) experiments revealed that AnAc 24:1ω5 inhibited E2-ERα interaction with the endogenous pS2 gene promoter region containing an ERE. These data indicate that AnAc 24:1ω5 inhibits cell proliferation, cell cycle progression and apoptosis in an ER-dependent manner by reducing ER-DNA interaction and inhibiting ER-mediated transcriptional responses.”
A 2013 publication Inhibition of PCAF by anacardic acid derivative leads to apoptosis and breaks resistance to DNA damage in BCR-ABL-expressing cells: Acetylation of histones and nonhistone proteins is a posttranslational modification which plays a major role in the regulation of intracellular processes involved in tumorigenesis. It was shown that different acetylation of proteins correlates with development of leukemia. It is proposed that histone acetyltransferases (HATs) are important novel drug targets for leukemia treatment, however data are still not consistent. Our previous data showed that a derivative of anacardic acid – small molecule MG153, which has been designed and synthesized to optimize the HAT inhibitory potency of anacardic acid, is a potent inhibitor of p300/CBP associated factor (PCAF) acetyltransferase. Here we ask whether inhibition of PCAF acetyltransferase with MG153 will show proapoptotic effects in cells expressing BCR-ABL, which show increased PCAF expression and are resistant to apoptosis. We found that inhibition of PCAF decreases proliferation and induces apoptosis, which correlates with loss of the mitochondrial membrane potential and DNA fragmentation. Importantly, cells expressing BCR-ABL are more sensitive to PCAF inhibition compared to parental cells without BCRABL. Moreover, inhibition of PCAF in BCR-ABL-expressing cells breaks their resistance to DNA damage-induced cell death. These findings provide direct evidence that targeting the PCAF alone or in combination with DNA-damaging drugs shows cytotoxic effects and should be considered as a prospective therapeutic strategy in chronic myeloid leukemia cells. Moreover, we propose that anacardic acid derivative MG153 is a valuable agent and further studies validating its therapeutic relevance should be performed.
A study published last month (June 2014) Discovery of Protein Disulfide Isomerase P5 Inhibitors that Reduce the Secretion of MICA from Cancer Cells points to another anti-cancer property of anacardic acid: “In order to regulate the activity of P5, which is a member of the protein disulfide isomerase family, we screened a chemical compound library for P5-specific inhibitors, and identified two candidate compounds (anacardic acid and NSC74859). Interestingly,anacardic acid inhibited the reductase activity of P5, but did not inhibit the activity of protein disulfide isomerase (PDI), thiol-disulfide oxidoreductase ERp57, or thioredoxin. NSC74859 inhibited all these enzymes. When we examined the effects of these compounds on the secretion of soluble major histocompatibility complex class-I-related gene A (MICA) from cancer cells, anacardic acid was found to decrease secretion. In addition, anacardic acid was found to reduce the concentration of glutathione up-regulated by the anticancer drug 17-demethoxygeldanamycin in cancer cells. These results suggest that anacardic acid can both inhibit P5 reductase activity and decrease the secretion of soluble MICA from cancer cells. It might be a novel and potent anticancer treatment by targeting P5 on the surface of cancer cells.”
Anacardic acids inhibits the SUMO pathway in AML cancer cells enhancing chemotherapies.
Another last-month publication was The ROS/SUMO Axis Contributes to the Response of Acute Myeloid Leukemia Cells to Chemotherapeutic Drugs: “Chemotherapeutic drugs used in the treatment of acute myeloid leukemias (AMLs) are thought to induce cancer cell death through the generation of DNA double-strand breaks. Here, we report that one of their early effects is the loss of conjugation of the ubiquitin-like protein SUMO from its targets via reactive oxygen species (ROS)-dependent inhibition of the SUMO-conjugating enzymes. Desumoylation regulates the expression of specific genes, such as the proapoptotic gene DDIT3, and helps induce apoptosis in chemosensitive AMLs. In contrast, chemotherapeutics do not activate the ROS/SUMO axis in chemoresistant cells. However, pro-oxidants or inhibition of the SUMO pathway by anacardic acid restores DDIT3 expression and apoptosis in chemoresistant cell lines and patient samples, including leukemic stem cells. Finally, inhibition of the SUMO pathway decreases tumor growth in mice xenografted with AML cells. Thus, targeting the ROS/SUMO axis might constitute a therapeutic strategy for AML patients resistant to conventional chemotherapies.”
Endoplasmic reticulum stress and autophagy
Most of the properties of anacardic acid listed above have been known for years. However, there are also important new discoveries. As our knowledge of molecular biology and pathways involved in health and disease states become better known, we are discovering properties of the acid that were unknown before. For example, effective autophagyis now regarded to be an important tool for inducing death of some strains of cancer cells. The 2014 publication Induction of the endoplasmic reticulum stress and autophagy in human lung carcinoma A549 cells byanacardic acidreports: “Anacardic acid (AA, 2-hydroxy-6-pentadecylbenzoic acid), a constituent of the cashew-nut shell, has a variety of beneficial effects on the treatment of cancer and tumors. However, the fact that AA induces ER stress and autophagy in cancer cell is not known. We investigated the effect of AA on ER-stress and autophagy-induced cell death in cancer cells. Because of our interest in lung cancer, we used the non-small cell lung adenocarcinoma A549 cells treated with 3.0 μg/ml of AA for this research. In this research we found that AA induces intracellular Ca(2+) mobilization and ER stress. AA induced the ER stress-inducing factors, especially IRE1α, and the hallmarks of UPR, Grp78/Bip and GADD153/CHOP. AA inhibited the expression of p-PERK and its downstream substrate, p-elF2α. We also demonstrated that AA induces autophagy. Up-regulation of autophagy-related genes and the appearance of autophagosome in transfected cells with green fluorescent protein (GFP)-LC3 and GFP-Beclin1 plasmids showed the induction of autophagy in AA-treated A549 cells. The morphological analysis of intracellular organelles by TEM also showed the evidence that AA induces ER stress and autophagy. For the first time, our research showed that AA induces ER stress and autophagy in cancer cells.”
Mitochondrial-mediated apoptosis in cancer cells
The 2013 publication Anacardic acid induces mitochondrial-mediated apoptosis in the A549 human lung adenocarcinoma cells relates: “Anacardic acid (AA) is a constituent of the cashew nut shell and is known as an inhibitor of nuclear factor-κB (NF-κB). We investigated the cytotoxicity of AA on cancer cells and more experiments to reveal the cell death mechanism focused on A549 lung adenocarcinoma cells for our interest in lung cancer. To examine the molecular mechanism of cell death in AA treated A549 cells, we performed experiments such as transmission electron microscopy caspase-independent apoptosis with no inhibition of cytotoxicity by pan-caspase inhibitor, Z-VAD-fmk, in A549 cells. Our results showed the possibility of mitochondrial-mediated apoptosis through the activation of apoptosis-inducing factor (AIF) and an intrinsic pathway executioner such as cytochrome c. This study will be helpful in revealing the cell death mechanisms and in developing potential drugs for lung cancer using AA. (TEM), western blot analysis, fluorescence-activated cell sorting (FACS), genomic DNA extraction and staining with 4′,6-diamidino-2-phenylindole (DAPI). For the first time we revealed that AA induces “
Anacardic acid may enhance the proliferation of ovarian cancer cells
Surprise! There is another side to the cancer story. As is sometimes the case with health-producing substances, it appears that anacardic acid can be used by some cancer cells to enhance their survival and proliferation. The June 2014 publication Anacardic Acidenhances the proliferation of human ovarian cancer cellsreports: “BACKGROUND: Anacardic acid (AA) is a mixture of 2-hydroxy-6-alkylbenzoic acid homologs. Certain antitumor activities of AA have been reported in a variety of cancers. However, the function of AA in ovarian cancer, to date, has remained unknown. METHODS: Ovarian cancer cell lines were exposed to AA, after which cell proliferation, apoptosis, invasion and migration assays were performed. Phalloidin staining was used to observe lamellipodia formation. Reverse transcription polymerase chain reaction (RT-PCR) and western blotting were used to assess the mRNA and protein expression levels of Phosphatidylinositol 3-kinase (PI3K), vascular endothelial growth factor (VEGF) and caspase 3. RESULTS: Our results showed that AA promotes ovarian cancer cell proliferation, inhibits late apoptosis, and induces cell migration and invasion, as well as lamellipodia formation. AA exposure significantly up-regulated PI3K and VEGF mRNA and protein expression, while, in contrast, it down-regulated caspase 3 mRNA and protein expression in comparison to untreated control cells. CONCLUSION: Taken together, our results demonstrate for the first time that AA may potentiate the proliferation, invasion, metastasis and lamellipodia formation in ovarian cancer cell lines via PI3K, VEGF and caspase 3 pathways.”
I suspect that there might be a non-linear dose-response involved here. This was a cell-level study, and it is not clear what the implications are for in-vivo administration of anacardic acid. In any event, this new result clouds the picture when it comes to the impact of anacardic acid on cancers.
Use asa radiation therapy sensitizer
We have already mentioned the 2006 publication Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation.
The 2011 publication Anacardic acid induces caspase-independent apoptosis and radiosensitizes pituitary adenoma cellsreports: “OBJECT: Pituitary adenomas, which are common intracranial tumors, are associated with significant patient morbidity due to hormone secretion or mass effect or as a complication of therapy. Epigenetic regulation has emerged as an important component of malignant tumor pathogenesis, although the contribution in the progression of benign pituitary tumors remains largely unexplored. The present study evaluates the effect of anacardic acid (6-pentadecyl salicylic acid), a natural histone acetyltransferase inhibitor, on pituitary adenoma cells. METHODS: The concentration- and time-dependent effects of anacardic acid on the viability of GH3 and MMQ pituitary adenoma cells were determined by 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell cycle phase distribution, protein expression, and percentage of apoptotic cells were assessed by flow cytometry and Western blotting. Colony forming assays were used to study the radiosensitizing effect of anacardic acid. RESULTS: The present study identifies a novel antiproliferative and cytotoxic effect of anacardic acid on pituitary adenoma cells. These effects were associated with an increase in poly([adenosine diphosphate]-ribose) polymerase cleavage, sub-G1 arrest, and annexin V staining, consistent with apoptotic cell death; however, the pancaspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-(O-methyl)-fluoromethylketone failed to reverse anacardic acid-induced cell death, suggesting a possible nonclassical apoptotic mechanism. Anacardic acid also reduced the expression of survivin and X-linked inhibitor of apoptosis protein, antiapoptotic proteins associated with cellular survival and radioresistance, and radiosensitized pituitary adenoma cells. CONCLUSIONS: These findings warrant further exploration of anacardic acid as a single agent or as an adjunct to radiation therapy for the treatment of pituitary tumors.”
Induction of developmental changes in global gene expression during developmental stages
This was demonstrated in the Plasmodium falciparum parasite, the one that causes malaria. The 2008 publication Histone acetyltransferasere inhibitor Anacardic Acid Causes Changes in Global Gene Expression during In Vitro Plasmodium falciparum Development: “To better understand the role of histone lysine acetylation in transcription in Plasmodium falciparum, we sought to attenuate histone acetyltransferase (HAT) activity using anacardic acid (AA). We showed that AA reversibly and noncompetitively inhibited the HAT activity of recombinant PfGCN5. To a lesser extent, AA inhibited the PfGCN5 activity in parasite nuclear extracts but did not affect histone deacetylase activity. AA blocked the growth of both chloroquine-sensitive and -resistant strains, with a 50% inhibitory concentration of ∼30 μM. Treatment of the parasites with 20 μM of AA for 12 h had no obvious effect on parasite growth or gross morphology but induced hypoacetylation of histone H3 at K9 and K14, but not H4 at K5, K8, K12, and K16, suggesting inhibition of the PfGCN5 HAT. Microarray analysis showed that this AA treatment resulted in twofold or greater change in the expression of 271 (∼5%) parasite genes in late trophozoites, among which 207 genes were downregulated. Cluster analysis of gene expression indicated that AA mostly downregulated active genes, and this gene pool significantly overlapped with that enriched for H3K9 acetylation. We further demonstrated by chromatin immunoprecipitation and real-time PCR that AA treatment reduced acetylation near the putative promoters of a set of downregulated genes. This study suggests that the parasiticidal effect of AA is at least partially associated with its inhibition of PfGCN5 HAT, resulting in the disturbance of the transcription program in the parasites.”
Anti-microbial and anti-fungal activities
Anacardic acid has wonderful antibacterial and anti fungal properties that have been known since 1969. It can kill helicobacter pylori in the stomach and heal gastritis or gastric ulcers caused by this bacteria. The most likely antibiotic mechanism of action is the fact that anacardic acid can act as an iontophore, an electrically charged molecule. See Selective ionophoric properties of anacardic acid (1995) It is a very good anti fungal as well.
Anacardic acid is effective against MRSA bacteria
Probably the most interesting application of anacardic acid is that it is very effective for resistant strains of Staph aureus (MRSA). These are staph bacteria that have evolved to be resistant to almost all conventional bacteria creating a significant threat to public health(ref). The 2003 publication Antibacterial Action of Anacardic Acids against Methicillin ResistantStaphylococcus aureus (MRSA)reports “The structural and antibacterial activity relationship of 6-alk(en)ylsalicylic acids, also known as anacardic acids, was investigated against Gram-positive bacteria, emphasizing the methicillin resistant Staphylococcus aureus ATCC 33591 (MRSA) strain. The unsaturation in the alkyl side chain is not essential in eliciting activity but is associated with increasing the activity. The antibacterial activity of methicillin against MRSA strains was significantly enhanced in combination with C12:0-anacardic acid, and the fractional inhibitory concentration index for this combination was calculated as 0.281. It appears that biophysical disruption of the membrane (surfactant property) is due to the primary response to their antibacterial activity, while biochemical mechanisms are little involved. The compounds possessing the similar log Pvalues exhibit similar activity.”
Also you can see the 2004 publication Synergistic effects of anacardic acids and methicillin against methicillin resistant Staphylococcus aureusand the 2008 publication Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillinresistant Staphylococcus aureus.
Further publications on anti-bacterial and anti-fungal properties of anacardic acid
Antimicrobial effects of anacardic acids (1969)
Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil (1991)
Bactericidal activity of anacardic acids against Streptococcus mutans and their potentiation(1993)
Structure-antibacterial activity relationships of anacardic acids (1993)
Antifungal activity of anacardic acid, a naturally occurring derivative of salicylic acid(1997)
Anti-Helicobacter pylori agents from the Cashew Apple(1999)
Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens(2007)
Quorum-sensing inhibition
Some or possibly most of the antibacterial effects of anacardic acid can be explained because of its ability to inhibit quorum sensing communications among the bacteria. Specifically, with regard to the actions of anacardic acid against MRSA, inhibition of quorum sensing compromises the communication tool the bacteria needs for colony formation . See the blog entries on Quorum Sensing, Part 1: quorum sensing inhibition via phytochemicals – a new approach against infectious diseases and Part 2 – Intra and inter-species molecular communications.
The 2013 publication Amphypterygium adstringensanacardic acidmixture inhibits quorum
sensing-controlled virulence factors of Chromobacterium violaceum and Pseudomonas aeruginosa reports: “BACKGROUND AND AIMS: Quorum sensing (QS) is a process of bacterial cell-cell communication that controls a large number of systems affecting pathogenicity. Interrupting this communication system can provide nonvirulent pathogenic bacteria. The aim of this study was to evaluate the anti-quorum sensing (anti-QS) potential of an anacardic acids mixture isolated from Amphipterygium adstringens, a medicinal plant known as “cuachalalate”, to prevent the onset of bacterial infections as an alternate to antibiotics. METHODS: Initially we investigated the anti-QS activity of A. adstringens hexane extract (HE) by the inhibition of violacein production in Chromobacterium violaceum. From the active HE, an anacardic acid mixture (AAM) was obtained. The anti-quorum sensing activity of AAM was investigated by the rhamnolipid and pyocyanin production constraint as well as decrease of elastase activity, all being quorum sensing-controlled virulence factors expressed in the pathogenic bacteria Pseudomonas aeruginosa. RESULTS: HE induced a 91.6% of inhibition of the violecin production at 55 μg/mL concentration, whereas AAM showed 94% of inhibition at 166 μg/mL. In both cases, inhibition of violacein production did not affect the viability of the bacterium. AAM inhibited pyocyanin (86% at 200 μg/mL) and rhamnolipid (91% at 500 μg/mL) production in a dose/response form and decrease the elastase (75% at 500 μg/mL) activity in P. aeruginosa without affecting its development. CONCLUSIONS: Because an anacardic acids mixture isolated from A. adstringens demonstrated anti-QS, it could be further exploited for novel molecules to treat the emerging infections of antibiotic-resistant bacterial pathogens.”
Use in dentistry
Anacardic acid derivatives might be useful for the prevention of dental cavities. The publication Design and evaluation of anacardic acid derivatives as anticavity agentsreports: “On the basis of antibacterial anacardic acids, 6-pentadecenylsalicylic acids, isolated from the cashew apple, Anacardium occidentale L. (Anacardiaceae), a series of 6-alk(en)ylsalicylic acids were synthesized and tested for their antibacterial activity against Streptococcus mutans ATCC 25175. — Among them, 6-(4′,8′-dimethylnonyl)salicylic acid was found to exhibit the most potent antibacterial activity against this cariogenic bacterium with the minimum inhibition concentration (MIC) of 0.78 μg/ml.” The antibacterial activity is probably associated with suppression of quorum sensing and bacterial colony formation.
Anti-gout properties
Anacardic acid inhibits xanthine oxidase, an important enzyme in the formation of uric acid. Gout is a condition where uric acid accumulates and precipitates in joints and in soft tissue, causing gouty arthritis. Hypoxanthine is converted into xanthine, which is then converted into uric acid. The enzyme that catalyzes the conversion of hypoxanthine to xanthine and then to uric acid is called Xanthine oxidase. Anacardic acid inhibits this enzyme.
The 2003 publication Characterization of xanthine oxidase inhibition by anacardic acidsrelates: “Anacardic acid, 6[8(Z), 11(Z), 14-pentadecatrienyl]salicylic acid, inhibits generation of superoxide radicals by xanthine oxidase. This inhibition does not follow a hyperbolic inhibition, depends on anacardic acid concentrations, but follows a sigmoidal inhibition. — The results indicate that anacardic acid binds to allosteric sites near the xanthine-binding domain in xanthine oxidase. Salicylic acid moiety and alkenyl side chain in anacardic acid are associated with the cooperative inhibition and hydrophobic binding, respectively.”
Anacardic acid may help protect lungs from air pollutants
The 2013 publication Anacardic Acids from Cashew Nuts Ameliorate Lung Damage Induced by Exposure to Diesel Exhaust Particles in Micereports: “Anacardic acids from cashew nut shell liquid, a Brazilian natural substance, have antimicrobial and antioxidant activities and modulate immune responses and angiogenesis. As inflammatory lung diseases have been correlated to environmental pollutants exposure and no reports addressing the effects of dietary supplementation with anacardic acids on lung inflammation in vivo have been evidenced, we investigated the effects of supplementation with anacardic acids in a model of diesel exhaust particle- (DEP-) induced lung inflammation. BALB/c mice received an intranasal instillation of 50 μg of DEP for 20 days. Ten days prior to DEP instillation, animals were pretreated orally with 50, 150, or 250 mg/kg of anacardic acids or vehicle (100 μL of cashew nut oil) for 30 days. The biomarkers of inflammatory and antioxidant responses in the alveolar parenchyma, bronchoalveolar lavage fluid (BALF), and pulmonary vessels were investigated. All doses of anacardic acids ameliorated antioxidant enzyme activities and decreased vascular adhesion molecule in vessels. Animals that received 50 mg/kg of anacardic acids showed decreased levels of neutrophils and tumor necrosis factor in the lungs and BALF, respectively. In summary, we demonstrated that AAs supplementation has a potential protective role on oxidative and inflammatory mechanisms in the lungs.”
Anacardic acid might be useful for reducing pain hypersensitivity after surgical incisions.
At least that is the implication of another newer (2013) study publication, Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. “BACKGROUND:
The regulation of gene expression in nociceptive pathways contributes to the induction and maintenance of pain sensitization. Histone acetylation is a key epigenetic mechanism controlling chromatin structure and gene expression. Chemokine CC motif receptor 2 (CXCR2) is a proinflammatory receptor implicated in neuropathic and inflammatory pain and is known to be regulated by histone acetylation in some settings. The authors sought to investigate the role of histone acetylation on spinal CXCR2 signaling after incision. METHODS: Groups of 5-8 mice underwent hind paw incision. Suberoylanilide hydroxamic acid and anacardic acid were used to inhibit histone deacetylase and histone acetyltransferase, respectively. Behavioral measures of thermal and mechanical sensitization as well as hyperalgesic priming were used. Both message RNA quantification and chromatin immunoprecipitation analysis were used to study the regulation of CXCR2 and ligand expression. Finally, the selective CXCR2 antagonist SB225002 was administered intrathecally to reveal the function of spinal CXCR2 receptors after hind paw incision. RESULTS: Suberoylanilide hydroxamic acid significantly exacerbated mechanical sensitization after incision. Conversely, anacardic acid reduced incisional sensitization and also attenuated incision-induced hyperalgesic priming. Overall, acetylated histone H3 at lysine 9 was increased in spinal cord tissues after incision, and enhanced association of acetylated histone H3 at lysine 9 with the promoter regions of CXCR2 and keratinocyte-derived chemokine (CXCL1) was observed as well. Blocking CXCR2 reversed mechanical hypersensitivity after hind paw incision.”
Anacardic acid is closely related to urushiol, the active toxin in poison ivy, poison oak and poison sumac – and exposing the skin to it may lead to contact dermatitis
Here is another down side, The 2012 publication Urushiol-induced contact dermatitis caused during Shodhana (purificatory measures) of Bhallataka (Semecarpus anacardium Linn.) fruitrelates: “Bhallataka (Semecarpus anacardium Linn.; Ancardiaceae) is mentioned under Upavisha group in Ayurvedic classics and it is described as a poisonous medicinal plant in Drugs and Cosmetics Act (India), 1940. Fruit of Bhallataka is used either as a single drug or as an ingredient in many compound formulations of Indian systems of medicine to cure many diseases. Tarry oil present in the pericarp of the fruit causes blisters on contact. The major constituent of the tarry oil is anacardic acid and bhilawanol, a mixture of 3-n-pentadec(en)yl catechols. Bhilawanol A and B are known as Urushiols, and also, anacardic acid is closely related to Urushiol. Urushiol-induced contact dermatitis is the medical name given to allergic rashes produced by the oil Urushiol. This paper deals with five case reports of contact dermatitis caused during different stages of Shodhana (purificatory measures) of Bhallataka fruit due to improper handling of the utensils and disposal of media used in Shodhana procedure and their Ayurvedic management. 00.” If you eat a cashew nut raw, it can burn your mouth!
Pharmaceutical companies have been exploring variants of Anacardic acid for the development of drugs.
As is often the case when there is a plant-based substance that has important biological effects, drug companies have been looking for biological analogs. The “good” reasons usually cited are that a substance is wanted with more powerful biological effects, better bioavailability and less side effects. Skeptics point out that often the “real” reason is that a molecule is wanted that can be patented and owned. No drug company is ready to spring for hundreds of millions of dollars for a clinical trial for an unpatentable natural substance. “There ain’t no money in something that grows in a plant and that anybody can sell cheap.” So, some of the studies mentioned are based on testing chemical analogs.
The 2010 publication Improved inhibition of the histone acetyltransferase PCAF by an anacardic acid derivativerelates: “Several lines of evidence indicate that histone acetyltransferases (HATs) are novel drug targets for treatment of diseases like, for example, cancer and inflammation. The natural product anacardic acid is a starting point for development of small molecule inhibitors of the histone acetyltransferase (HAT) p300/CBP associated factor (PCAF). In order to optimize the inhibitory potency, a binding model for PCAF inhibition by anacardic acid was proposed and new anacardic acid derivatives were designed. Ten new derivatives were synthesized using a novel synthetic route. One compound showed a twofold improved inhibitory potency for the PCAF HAT activity and a twofold improved inhibition of histone acetylation in HEP G2 cells.”
The 2007 publication Molecular design of anti-MRSA agents based on the anacardic acid scaffolddescribes such a search for analogs for anacardic acid: “A series of anacardic acid analogues possessing different side chains viz. phenolic, branched, and alicyclic were synthesized and their antibacterial activity tested against methicillin-resistant Staphylococcus aureus(MRSA). The maximum activity against this bacterium occurred with the branched side-chain analogue, 6-(4′,8′-dimethylnonyl)salicylic acid, and the alicyclic side-chain analogue, 6-cyclododecylmethyl salicylic acid, with the minimum inhibitory concentration (MIC) of 0.39 μg/mL, respectively. This activity was superior to that of the most potent antibacterial anacardic acid isolated from the cashew Anacardium occidentale (Anacardiaceae), apple and nut, that is, the 6-[8′(Z),11′(Z),14′-pentadecatrienyl]salicylic acid.” Also, there is the 2013 publication Efficient synthesis ofanacardic acidanalogues and their antibacterial activities.
Looking forward
The molecular actions and health-producing properties of anacardic acid documented here seem amazing, but there appears to be a lot more we don’t know about in-vivo actions of the substance. For example, after building a very convincing case for its anti-cancer activities above, we cited a publication that indicates that anacardic acid may enhance the “proliferation, invasion, metastasis and lamellipodia formation in ovarian cancer cell lines.” What gives? I expect we will continue to hear more about it as time goes on.



Would it be advisable at this time to take either anacardic acid or CNSL as a supplement? If so, where is a source, and at what dosage and frequency?
Mike Yaros