By Vince Giuliano
We have discussed important aspects of cell tissue regeneration in previous blog entries. In particular see AGING, CELL AND TISSUE REPAIR, RENEWAL AND REGENERATION, INFLAMMATION AND THE SASP. The present blog entry deals with circadian regulation; in particular with regulation of cell tissue regeneration. Circadian regulation is of very great importance both for understanding the mechanisms involved and determining the patterns of rest and sleep that are essential if effective repair and renewal are to take place.  Further, I explore some practical things we can do to make sure our body clocks are fully supportive of initiatives we may take to further cell and tissue renewal and regeneration. I include timing suggestions for exercise, meals and taking supplements, and assuring nightly periods of restorative deep and REM sleep. Image source
Further, I explore some practical things we can do to make sure our body clocks are fully supportive of initiatives we may take to further cell and tissue renewal and regeneration. I include timing suggestions for exercise, meals and taking supplements, and assuring nightly periods of restorative deep and REM sleep. Image source
We have previously published two blog entries related to this one, the 2014 entry Shedding new light on circadian rhythms, and the 2012 entry by Victor Circadian Regulation,NMN, Preventing Diabetes, and Longevity. This was a very prescient publication, pointing out the probable relevancy of NAD+ augmentation years before this subject became popular. These publications contain much background information relevant to this blog entry.
I proceed by reviewing:
- Relevance of circadian regulation of cell and tissue repair, renewal and regeneration processes.
- Basic circadian regulatory processes – how they work and their complexities
- Consequences of improper circadian clock functioning: diseases and aging
- Some opinions about how older people can best respect the circadian regulatory processes in the interests of their health and longevity
- A bit on sleep monitoring with wearables
- An update on key actions of a traditional circadian regulator – Melatonin
HOW IMPORTANT IS CIRCADIAN REGULATION?
Very. Most mammals – us humans included – have evolved in circumstances of day-night cycles, and many of our internal operations have been optimized during our long periods of evolution to function differently during periods of the daily cycle. Sleepiness, alertness, hunger, endurance strength, muscle coordination, sexual arousal and mental acuity are among the list of matters we have learned to be so regulated. And we have learned about the importance of sleep and rest. But behind the obvious effects are hundreds of pathways affected by circadian cycle signaling, in one way or another.
There are two master body clocks and numerous peripheral ones. As explained in the introduction to the May 2019 publication Circadian Regulation in Tissue Regeneration: “Earth’s 24 h rotation around its axis has influenced organismal development to be centered around cyclic patterns of day- and night-time function. Circadian systems are determined by environmental zeitgebers, or time givers, that entrain clock rhythmicity. In mammals, the most prominent zeitgebers are the onset of light and darkness, though other factors such as food intake and temperature can influence clock mechanisms [1,2]. The circadian system consists of both central and peripheral clocks. The central clock in mammals stems from a network of neurons in the suprachiasmatic nucleus (SCN); these neurons, in addition to maintaining their own cell-intrinsic clock, receive photic cues from the retina to synchronize peripheral day and night cycles throughout the body using a variety of mechanisms, including nervous system signaling, body temperature regulation, hormonal signaling, and regulation of metabolism [2,3]. Peripheral tissue circadian rhythms are synchronized by the central clock, but they also contain their own cell-intrinsic circadian rhythms [4]. As evidence, cells in culture also retain cell-autonomous 24 h rhythmicity, although synchronization is required for detection at the whole culture level [5]. — In mammalian cells, peripheral clocks are maintained by two major auto-regulatory feedback loops that involve transcription of master regulator genes Clock and Bmal1. Clock and Bmal1 proteins heterodimerize and bind to enhancer E-box regions that promote transcription of thousands of genes throughout the body; 43% of protein encoding genes show circadian oscillations in expression in at least one organ [6,7,8,9]. Transcriptional targets include negative circadian master regulator genes Per1, Per2, Per3, Cry1, and Cry2 [6,10]. The first auto-regulatory feedback loop is formed when Per and Cry proteins accumulate in the cytoplasm. Eventually, Cry/Per heterodimers translocate to the nucleus and bind the Clock/Bmal1 complex, inhibiting further Cry/Per transcription as well as the transcription of other genes regulated by the Clock/Bmal1 complex [7,8,11,12,13]. Cry and Per proteins are eventually ubiquitinated and degraded, allowing for another rise in Clock/Bmal1 activity [14,15].” OK. This is starting to get complicated. Welcome to the real world of biology!
Here are some diagrams listing what might be expected to go on during a day of circadian regulation.
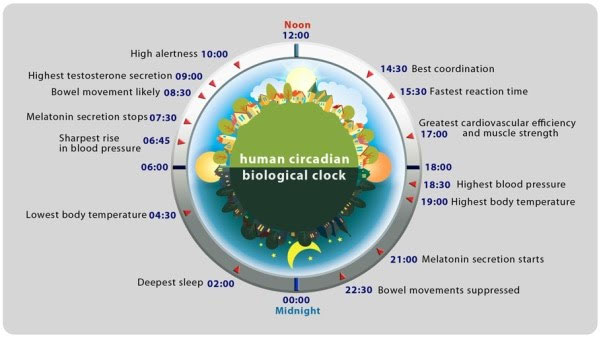
Here is an
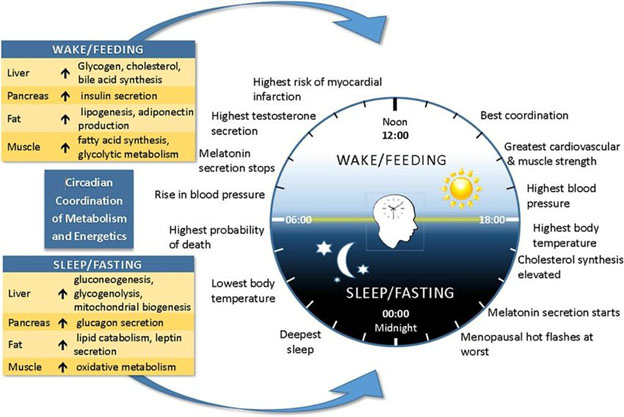
other model of what might typically be expected to go on around the clock:
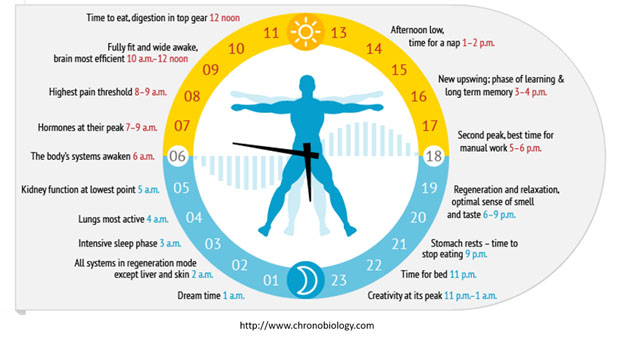
Here is yet another portrayal of chronobiological events. You will note that each clock portrays different things.
If you want to see many-more body processes that are circadian-regulated, here is a large additional collection of relevant images.
History of circadian regulation
Circadian regulation of biological species is extremely ancient, apparently going back to the times when the heavy cloud cover over all of the earth started to lift and day and night started to become distinct from each other. The first oxygen-synthesizing bacteria appeared in our oceans between 3 and 4 billion years ago. Before that we had no oxygen in the atmosphere(ref). But is was not until approximately a billion years ago that we started to see circadian regulation, initially in cyanobacteria and related species. The 2003 publication Origin and evolution of circadian clock genes in prokaryotes discusses this early history: “Circadian clock genes are a vital and essential feature of eukaryotes (1). Cyanobacteria were the first prokaryotes reported to have the circadian clock regulated by a cluster of three genes: kaiA, kaiB, and kaiC (2). Cyanobacteria are among the oldest organisms on the earth, and they are among the most successful in terms of ecological plasticity and adaptability (3). In adaptation strategy of cyanobacteria, circadian clock genes are of particular importance, because they underlie fundamental physiological processes such as the regulation of nitrogen fixation, cell division, and photosynthesis (4). The clock genes are ubiquitous in cyanobacteria (5). In most cyanobacteria, these genes were reported as a single copy (2, 5), although some of them (kaiB; ref. 6) or even a whole cluster (7) may be duplicated. They were shown to operate as a single unit and to follow a feedback model of regulation: kaiA positively affects kaiBC promoter, whereas overexpression of kaiC represses it (2, 8). Among these genes, kaiC is a crucial component of clock precession in cyanobacteria (9–11). — In this study we reconstruct the origin and evolutionary patterns of the circadian clock genes in prokaryotes. Using available sequences from the public databases, we performed extensive phylogenetic analysis of the kai genes. The results suggest that the three prokaryotic circadian pacemakers have quite different evolutionary histories, and two of them, kaiA and kaiB, originated in cyanobacteria. The three-gene kaiABC cluster itself evolved ≈1,000 Mya.”

(Image source). Cyanobacteria bloom on lake
So that’s how it got started, and the circadian regulatory genes evolved as did species. Fast forwarding to mammals starting a mere 300 million years ago or so, we find that our circadian regulatory genes are virtually the same as the ones operating in mice, skunks and zebras. The 2003 publication Clocks, genes and sleep reports: “In mammals, circadian rhythms are governed by a paired master oscillator in the suprachiasmatic nuclei (SCN), which controls slave oscillators throughout the body.2,3 The SCN determines the individual’s free-running circadian period, τ,4 which in man is reported to average 24.2 h in the absence of light cues.5 Our laboratory has observed a range of 23.83-25.00 h in blind individuals.6 Variability within the species, whereby individuals reach peak performance at different times of day, may have conveyed an evolutionary advantage to early human societies. In a normal individual the phase of the SCN oscillator is entrained each day to the external light/dark cycle by information relayed through the retinohypothalamic tract.7,8 — In vertebrates, sleep has evolved as a sustained period of physical inactivity during a specific part of the circadian cycle.9 The distribution of rest and activity between the night and the day varies between taxa. Our reptilian ancestors, being exothermic, were active during the day (diurnal), but the endothermic mammals that radiated from these species were nocturnal. From there, various mammals (including most primates) reverted to diurnality through secondary modifications, notably in the visual system. The retina was recolonized by color-sensitive cones (not required for night vision) and the higher visual canters were rewired.9–13 — The parameters within the circadian system that define nocturnality and diurnality are only partly understood.14 As a general rule, nocturnal species have a τ<24 hours, diurnal species have a τ>24 hours.15 In addition, the composition of visual photoreceptors seems important. People who are color blind through complete lack of cone photoreceptors tend to be photophobic, and on the Micronesian island of Pingelap, where the condition is common, some affected individuals specialize in night fishing.16”
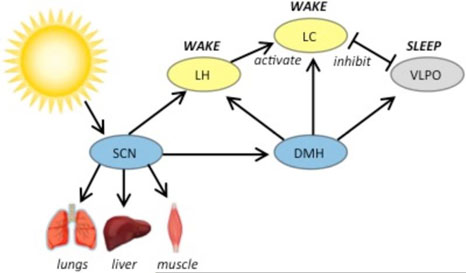
Image source “Sleep and circadian brain circuitry in mammals — The SCN is the central circadian pacemaker, entraining other brain regions as well as peripheral tissues. The SCN projects directly to the wake-promoting LH, which contains hypocretin neurons, and to the DMH. The DMH projects broadly to sleep and arousal centers. Abbreviations: SCN: suprachiasmatic nucleus; DMH: dorsomedial hypothalamus; LH: lateral hypothalamus; LC: locus coeruleus; VLPO: ventrolateral preoptic area”
CIRCADIAN REGULATION OF REGENERATIVE PROCESSES
Many key regenerative processes are subject to circadian regulation. The May 2019 publication Circadian Regulation in Tissue Regeneration states the case in point. “Circadian rhythms regulate over 40% of protein-coding genes in at least one organ in the body through mechanisms tied to the central circadian clock and to cell-intrinsic auto-regulatory feedback loops. Distinct diurnal differences in regulation of regeneration have been found in several organs, including skin, intestinal, and hematopoietic systems. Each regenerating system contains a complex network of cell types with different circadian mechanisms contributing to regeneration. In this review, we elucidate circadian regeneration mechanisms in the three representative systems. We also suggest circadian regulation of global translational activity as an understudied global regulator of regenerative capacity. A more detailed understanding of the molecular mechanisms underlying circadian regulation of tissue regeneration would accelerate the development of new regenerative therapies. — Circadian rhythms from both central and peripheral clock mechanisms have been found to influence efficacy of regeneration of many different tissues. Among the many cell types involved in regeneration, stem cells have varied circadian rhythmicity depending on differentiation state, with an extreme example being the lack of master regulator rhythmicity in pluripotent stem cells. Reflecting the current interest in stem cell biology, circadian regulation of stem cell activity has been comprehensively reviewed in recent articles [18,19]. Another widely studied area, circadian gating of cell cycle progression at multiple checkpoints, including the G1-S and the G2-M transitions, has also been extensively studied and reviewed, both in physiological tissues and in the context of carcinogenesis [20,21,22,23,24,25,26]. Therefore, in this review, we highlight circadian regulation of stem cell biology, cell cycle, and other cellular functions from the perspective of regeneration in three specific organs: skin, intestine, and blood (Figure 1). These representative tissues demonstrate time of day-dependent differences in regenerative capacity, an understudied but important contributor during wound healing. We also propose that circadian fluctuations of global translational activity may affect the regenerative capacity at any given time of day and should be taken into consideration in future studies of regeneration.”
The 2017 publication Circadian clocks: from stem cells to tissue homeostasis and regeneration provides another partial summary: “The circadian clock is an evolutionarily conserved timekeeper that adapts body physiology to diurnal cycles of around 24 h by influencing a wide variety of processes such as sleep‐to‐wake transitions, feeding and fasting patterns, body temperature, and hormone regulation. The molecular clock machinery comprises a pathway that is driven by rhythmic docking of the transcription factors BMAL1 and CLOCK on clock‐controlled output genes, which results in tissue‐specific oscillatory gene expression programs. Genetic as well as environmental perturbation of the circadian clock has been implicated in various diseases ranging from sleep to metabolic disorders and cancer development. Here, we review the origination of circadian rhythms in stem cells and their function in differentiated cells and organs. We describe how clocks influence stem cell maintenance and organ physiology, as well as how rhythmicity affects lineage commitment, tissue regeneration, and aging.”
Continuing with material from the May 2019 publication Circadian Regulation in Tissue Regeneration: “Levels of the Clock/Bmal1 complex are regulated by a second auto-regulatory feedback loop that affects transcription of Bmal1. Clock/Bmal1 complexes induce expression of nuclear receptor transcriptional activators RORα (retinoic acid receptor-related orphan receptor α) and RORβ, and repressors Rev-ErbAα (reverse c-erbAα) and Rev-ErbAβ. Bmal1 transcription is affected by competitive binding of these two nuclear receptors to Rev-ErbA/ROR response elements (RREs) in the Bmal1 promoter region. Rev-Erbs inhibit Bmal1 expression, while RORs promote Bmal1 expression as essential components to stabilize circadian rhythmicity [7,8,16,17]. A variety of chromatin-modifying enzymes, kinases, phosphatases, and RNA-binding factors also modify these core master regulators to ensure circadian rhythmicity [7,8]. Circadian rhythms from both central and peripheral clock mechanisms have been found to influence efficacy of regeneration of many different tissues. Among the many cell types involved in regeneration, stem cells have varied circadian rhythmicity depending on differentiation state, with an extreme example being the lack of master regulator rhythmicity in pluripotent stem cells. Reflecting the current interest in stem cell biology, circadian regulation of stem cell activity has been comprehensively reviewed in recent articles [18,19]. Another widely studied area, circadian gating of cell cycle progression at multiple checkpoints, including the G1-S and the G2-M transitions, has also been extensively studied and reviewed, both in physiological tissues and in the context of carcinogenesis [20,21,22,23,24,25,26]. Therefore, in this review, we highlight circadian regulation of stem cell biology, cell cycle, and other cellular functions from the perspective of regeneration in three specific organs: skin, intestine, and blood (Figure 1). These representative tissues demonstrate time of day-dependent differences in regenerative capacity, an understudied but important contributor during wound healing. We also propose that circadian fluctuations of global translational activity may affect the regenerative capacity at any given time of day and should be taken into consideration in future studies of regeneration.”
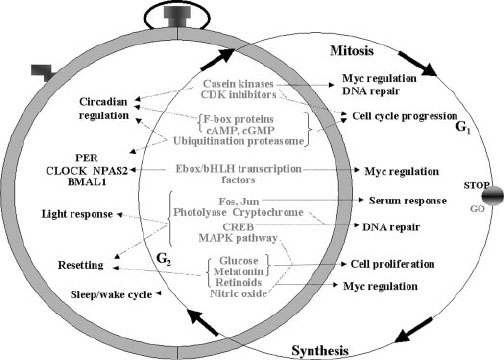
Common elements shared by biological clocks and cell cycle. Image source. Myc is a broadly acting regulator of transcriptional activities of families of genes involved in both healthy and cancer processes. Myc binds to something like 25,000 gene sites, and there is much literature related to it(ref).
If you are interested in the molecular biology of clock regulation and the multiple pathways involved, I suggest you delve into the above publications and others they link to.
CIRCADIAN REGULATION AND THE NAD WORLD
Jim Watson and I have devoted a series of blog entries to metabolic events in the “NAD WORLD.” (ref)(ref)(ref)(ref)(ref). These outline the compelling story of how expression of NAD+ declines with age, the numerous deleterious consequential effects, and what might be done to counter some of them in the interest of health and longevity. You can also view my 2018 PowerPoint presentation TALES OF NAD+. And again, relevant to NAD and circadian regulation, you can review Victor’s 2012 blog entry Circadian Regulation,NMN, Preventing Diabetes, and Longevity.
Relevant in the present context is the fact that events in the NAD salvage cycle are under circadian regulation, and also that these NAD salvage cycle events in turn impact on the circadian cycle. The 2010 publication “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging tells a part of the story: “SIR2 (silent information regulator 2) proteins, now called “sirtuins,” are an evolutionarily conserved family of NAD-dependent protein deacetylases/ADP-ribosyltransferases. Sirtuins have recently attracted major attention in the field of aging research, and it has been demonstrated that SIR2 and its orthologs regulate aging and longevity in yeast, worms, and flies. In mammals, the SIR2 ortholog SIRT1 coordinates important metabolic responses to nutritional availability in multiple tissues. Most recently, it has been demonstrated that SIRT1 regulates the amplitude and the duration of circadian gene expression through the interaction and the deacetylation of key circadian clock regulators, such as BMAL1 and PER2. More strikingly, we and others have discovered a novel circadian clock feedback loop in which both the rate-limiting enzyme in mammalian NAD biosynthesis, nicotinamide phosphoribosyltransferase (NAMPT), and NAD levels display circadian oscillations and modulate CLOCK:BMAL1-mediated circadian transcriptional regulation through SIRT1, demonstrating a new function of NAD as a “metabolic oscillator.” These findings reveal a novel system dynamics of a recently proposed systemic regulatory network regulated by NAMPT-mediated NAD biosynthesis and SIRT1, namely, the NAD World. In the light of this concept, a new connection between physiological rhythmicity, metabolism, and aging will be discussed.”
Regular readers of this blog may note that SIRT1 exercises a number of important functions related to health and longevity including in DNA repair, mitochondrial health and the control of chronic inflammation, so the close interaction of circadian events and the NAD salvage cycle (which impacts on the expression of SIRT1) is extremely important.
ORGAN SPECIFICITY OF CIRCADIAN RENEWAL PROCESSES
As mentioned above, there are numerous secondary and tertiary organ and cell-level clocks that roughly synchronize to the master clocks. Likewise, there are overall body circadian impacts and ones that are specific to particular organs and ones that are applicable to specific cell types. I can’t cover these all here but will refer to publications related to the intestinal track and the cornea of eyes as examples.
Gastrointestinal Track
Regenerative events in the gastrointestinal track are subject to strong circadian regulation, no surprise given the relative regularity of our eating and movement patterns. The 2017 publication The Circadian Clock Gene BMAL1 Coordinates Intestinal Regeneration offers this image and reports in summary:
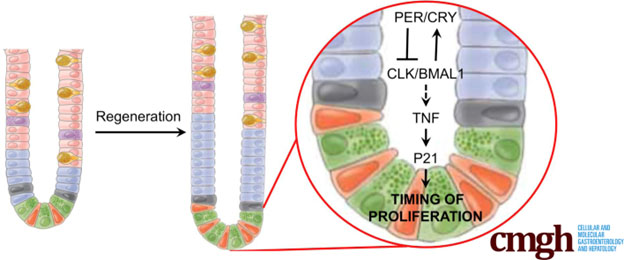
“BACKGROUND & AIMS: The gastrointestinal syndrome is an illness of the intestine caused by high levels of radiation. It is characterized by extensive loss of epithelial tissue integrity, which initiates a regenerative response by intestinal stem and precursor cells. The intestine has 24-hour rhythms in many physiological functions that are believed to be outputs of the circadian clock: a molecular system that produces 24-hour rhythms in transcription/translation. Certain gastrointestinal illnesses are worsened when the circadian rhythms are disrupted, but the role of the circadian clock in gastrointestinal regeneration has not been studied. — METHODS: We tested the timing of regeneration in the mouse intestine during the gastrointestinal syndrome. The role of the circadian clock was tested genetically using the BMAL1 loss of function mouse mutanj in vivo, and in vitro using intestinal organoid culture. — RESULTS: The proliferation of the intestinal epithelium follows a 24-hour rhythm during the gastrointestinal syndrome. The circadian clock runs in the intestinal epithelium during this pathologic state, and the loss of the core clock gene, BMAL1, disrupts both the circadian clock and rhythmic proliferation. Circadian activity in the intestine involves a rhythmic production of inflammatory cytokines and subsequent rhythmic activation of the JNK stress response pathway. — CONCLUSIONS: Our results show that a circadian rhythm in inflammation and regeneration occurs during the gastrointestinal syndrome. The study and treatment of radiation-induced illnesses, and other gastrointestinal illnesses, should consider 24-hour timing in physiology and pathology.”
Stem cell activities are also to various extents regulated by circadian factors. Again, in the case of intestines the 2019 publication Time after time: circadian clock regulation of intestinal stem cells reports: “Daily fluctuations in animal physiology, known as circadian rhythms, are orchestrated by a conserved molecular timekeeper, known as the circadian clock. The circadian clock forms a transcription-translation feedback loop that has emerged as a central biological regulator of many 24-h processes. Early studies of the intestine discovered that many digestive functions have a daily rhythm and that intestinal cell production was similarly time-dependent. As genetic methods in model organisms have become available, it has become apparent that the circadian clock regulates many basic cellular functions, including growth, proliferation, and differentiation, as well as cell signaling and stem cell self-renewal. Recent connections between circadian rhythms and immune system function, and between circadian rhythms and microbiome dynamics, have also been revealed in the intestine. These processes are highly relevant in understanding intestinal stem cell biology. Here we describe the circadian clock regulation of intestinal stem cells primarily in two model organisms: Drosophila melanogaster and mice. Like all cells in the body, intestinal stem cells are subject to circadian timing, and both cell-intrinsic and cell-extrinsic circadian processes contribute to their function.”
The Eye – Corneal Epithelium
The eye is another organ where issue renewal is understandably very susceptible to circadian regulation. Sensible, since we are not using our eyes while sleeping making that a good time for repair and renewal. The 2018 publication Ocular Clocks: Adapting Mechanisms for Eye Functions and Health reports; “Vision is a highly rhythmic function adapted to the extensive changes in light intensity occurring over the 24-hour day. This adaptation relies on rhythms in cellular and molecular processes, which are orchestrated by a network of circadian clocks located within the retina and in the eye, synchronized to the day/night cycle and which, together, fine-tune detection and processing of light information over the 24-hour period and ensure retinal homeostasis. Systematic or high throughput studies revealed a series of genes rhythmically expressed in the retina, pointing at specific functions or pathways under circadian control. Conversely, knockout studies demonstrated that the circadian clock regulates retinal processing of light information. In addition, recent data revealed that it also plays a role in development as well as in aging of the retina. Regarding synchronization by the light/dark cycle, the retina displays the unique property of bringing together light sensitivity, clock machinery, and a wide range of rhythmic outputs. Melatonin and dopamine play a particular role in this system, being both outputs and inputs for clocks. The retinal cellular complexity suggests that mechanisms of regulation by light are diverse and intricate. In the context of the whole eye, the retina looks like a major determinant of phase resetting for other tissues such as the retinal pigmented epithelium or cornea. Understanding the pathways linking the cell-specific molecular machineries to their cognate outputs will be one of the major challenges for the future.”
The 2017 publication Modulation of Circadian Rhythms Affects Corneal Epithelium Renewal and Repair in Mice reports: “PURPOSE: In mammalian corneal epithelium, mitosis shows a distinct circadian pattern. However, how this circadian pattern is maintained, and how it or its disruption influence renewal and regeneration remain unclear. — METHODS: C57BL/6 mice were maintained under 12-hour light/12-hour dark (LD), 12-hour light/12-hour light (LL), 12-hour dark/12-hour dark (DD), or reversed LD (DL, 12-hour dark/12-hour light; jet-lag defined as a shift of 12 hours) conditions. Mitotic cells in corneal epithelium were enumerated and analyzed via immunofluorescence at different zeitgeber times (ZTs). Expression of core clock genes (Clock, Bmal1, Period2, Cry1, and Rev-erbα) was qualified via quantitative RT-PCR. The rate and quality of healing at different ZT times and after administration of two small-molecule modifiers of the circadian clock, KL001 and SR8278, was evaluated. — RESULTS: In this study, photic cues were found to influence the 24-hour rhythm of corneal clock gene expression and epithelial cell mitosis in mice. Disruption of the circadian clock by exposure to constant light, constant dark, or jet-lag conditions modified the normal 24-hour patterns of corneal epithelial mitosis and corneal clock gene expression. The time of day of wound occurrence affected the rate and quality of corneal healing, with both of these parameters peaking during the more mitotically active hours of the morning. The two small-molecule modifiers of the circadian clock, KL001 and SR8278, had negative and positive effects on corneal wound healing, respectively. — CONCLUSIONS: Circadian rhythms significantly influence corneal epithelium renewal and repair in mice. Our findings reveal possible opportunities for biological rhythm-based interventional strategies to control corneal healing and restore corneal homeostasis.”
THE CIRCADIAN CLOCK AND INFLAMMATION
I have written extensively about the jmportance of controlling chronic inflammation for general health and longevity. But what are the chronobiological properties of inflammation? When in the course of 25 hours is it most and least likely to be manifest?. And specifically, if one takes anti-inflammatory herbs or our liposomal herbal product 4 Herb Synergy to control inflammation, when is the best time of day to do this? Some applicable insights can be found in the 2016 publication The circadian clock regulates inflammatory arthritis. “There is strong diurnal variation in the symptoms and severity of chronic inflammatory diseases, such as rheumatoid arthritis. In addition, disruption of the circadian clock is an aggravating factor associated with a range of human inflammatory diseases. To investigate mechanistic links between the biological clock and pathways underlying inflammatory arthritis, mice were administered collagen (or saline as a control) to induce arthritis. The treatment provoked an inflammatory response within the limbs, which showed robust daily variation in paw swelling and inflammatory cytokine expression. Inflammatory markers were significantly repressed during the dark phase. Further work demonstrated an active molecular clock within the inflamed limbs and highlighted the resident inflammatory cells, fibroblast-like synoviocytes (FLSs), as a potential source of the rhythmic inflammatory signal. Exposure of mice to constant light disrupted the clock in peripheral tissues, causing loss of the nighttime repression of local inflammation. Finally, the results show that the core clock proteins cryptochrome (CRY) 1 and 2 repressed inflammation within the FLSs, and provide novel evidence that a CRY activator has anti-inflammatory properties in human cells. We conclude that under chronic inflammatory conditions, the clock actively represses inflammatory pathways during the dark phase. This interaction has exciting potential as a therapeutic avenue for treatment of inflammatory disease.— The circadian clock is an internal timing mechanism that enables organisms to anticipate rhythmic daily changes in the environment and adapt their physiology accordingly. At a molecular level, 24-h oscillations are created by a transcriptional–translational feedback loop (1). Within this loop, the positive transcriptional regulators CLOCK and BMAL drive the transcription of the negative regulators period (per) and cryptochrome (cry). The PER and CRY proteins dimerize and inhibit CLOCK/BMAL-mediated transcription. The PER/CRY complex is subsequently degraded, releasing this inhibitory effect and allowing a new cycle of transcription to begin. Immune system function and activity are strongly influenced by the circadian clock, under both homeostatic conditions and during inflammatory challenge (2). Much of this temporal control of immune responses is orchestrated by peripheral clocks—timers within individual cellular components of the immune system that confer rhythmicity to their functionality. –’
“Rheumatoid arthritis (RA) is a debilitating autoimmune condition, characterized by inflammation and swelling within the joints. Inflammation within the joint is driven both by resident synovial cells [fibroblast-like synoviocytes (FLSs)] and by infiltrating immune cells (including monocytes, macrophages and T cells). I n addition to acute swelling and pain, this chronic inflammatory state leads to joint remodelling, causing severe disability. RA shows daily variations in symptom and sign severity (12). Clinical studies dating back to the 1960s document patients with RA exhibiting increased joint stiffness in the morning (13), and it is well established that immunologic disease markers associated with RA show time-of-day variation. In particular, IL6 has been identified as strongly rhythmic in patients with RA, with elevated levels in the morning (14, 15). However, despite this clear clinical evidence of rhythmic disease presentation, little is known about how the pathophysiology of RA is governed by the circadian clock.”
So, circadian activation of CRY proteins minimize the inflammatory state at night. The FLS cells are most clock-active in the day, leading to the swelling, joint siffness, disability, pain and tissue remodeling mischief characteristic of RA inflammation. I infer therefore that the best time to take anti-inflammatory supplements like 4 Herb Synergy to avoid such damage is morning, and that is what I personally do.
WHEN OUR CLOCKS GO SCREWY, WHAT CAN HAPPEN?
Our circadian rhythms are subject to disruption by a number of lifestyle factors including late or irregular shift working, travel across multiple time zones, irregular meal times, large ill-timed meals, excess alcohol, exposure to bright lights at night, disturbing news at bedtime, and late partying. This 2018 review article identifies the major risks and associated factors related with disruption in circadian system and sleep: Health risks associated with genetic alterations in internal clock system by external factors. “ — Adverse impacts of circadian dysrhythmia on body and mind are linked with lifestyle or routines such as frequently flying, working in repeatedly changing shifts and exposure to irregular light-dark conditions. Working around the clock and jetting around the globe are becoming necessary in our globalized world in some high-profile services. C rossing multiple time zones results in “jet lag,” a circadian rhythm disorder, that disturbs spatial cognition and hippocampal neurogenesis. Jet lag immediately causes fatigue and indigestion while chronic exposure to jet lag has strong impacts on spatial cognition and temporal lobe5. Externally induced disruption in natural sleep-wake cycles6and circadian rhythms is associated with neuronal dysfunction7,8, increased risk for psychiatric, cardiovascular and metabolic diseases3, and affects bone health9. — Long-term exposure to irregular dark-light cycles increases the risk of sleep disorders, metabolic disorders, mental abnormalities, depressive disorders, and hormonal abnormalities, that further develop cancer, gastrointestinal, and reproductive disorders10. Trans meridian travel may develop general malaise, headaches, insomnia, daytime sleepiness, impaired cognitive or physical performance and gastrointestinal disturbances. Impacts of shift-work on sleep duration quality can disrupt the physiological rhythms via desynchronization between hormonal rhythms, metabolic function and sleep cycle4. Physiological clock alterations 11 increase the risk of peptic ulcer and diabetes12–14.
Some of the problems are directly sleep-related, as illustrated here.
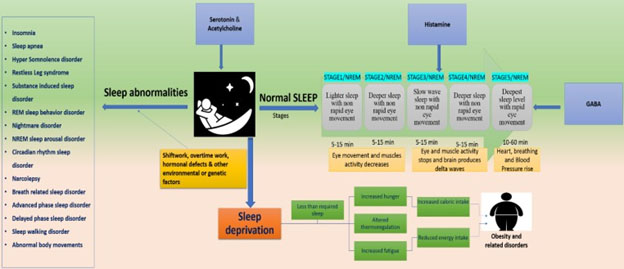
Five stages of normal sleep are shown along with how long they may typically last. All of these stages are needed for best rest and regeneration. My Oura ring, as described below, will tell me every morning how much time I spent in light sleep, deep sleep and REM sleep. So will my Fitbit Charge 3 smartwatch, although with less accuracy
In addition, numerous other negative consequences can be associated with circadian dysregulation as portrayed in this diagram.
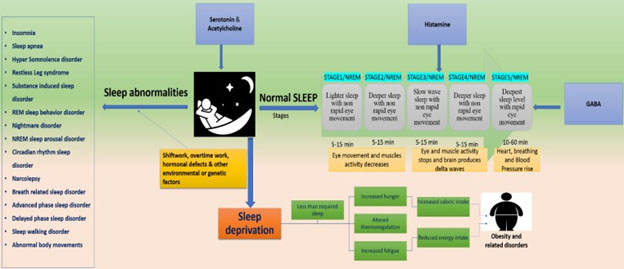
In addition, there are of course also numerous internal factors that can contribute to clock dysregulation, including conditions that can lead to insomnia(ref):
“Examples of medical conditions that can cause insomnia are:
- Nasal/sinus allergies
- Gastrointestinal problems such as reflux
- Endocrine problems such as hyperthyroidism
- Arthritis
- Asthma
- Neurological conditions such as Parkinson’s disease
- Chronic pain
- Low back pain
- Some medications such as those taken for the common cold and nasal allergies, high blood pressure, heart disease, thyroid disease, birth control, asthma, and depression can also cause insomnia.
- Restless legs syndrome”
SIMPLE THINGS TO DO TO KEEP OUR CLOCKS RUNNING WALL
The common-sense suggestion for those of us who are elderly or beset with diseases that disrupt our chronobiological patterns is of course a) develop awareness of our daily rhythms, b) try to the greatest extent possible to maintain consistent daily patterns as relate to sleep and awakeness, exercise and meals, and c) seek to schedule our activities at optimal times during the day given the operation of our natural clocks, d) avoid known disruptors of circadian thythms like bright or blue lights at night or late meals, and e) recognize that there are many different inputs we can make to the circadian clocks to give us better results.
- c) Scheduling our activities at optimal times.
There appears to be much conventional wisdom at to timing, but relatively little actual research. Morning is often thought to be best for learning by the elderly, This publication which appeared last month suggests that for motor skills, evening training is better; A Delayed Advantage: Multi-Session Training at Evening Hours Leads to Better Long-Term Retention of Motor Skill in the Elderly. “The acquisition and retention of motor skills is necessary for everyday functioning in the elderly and may be critical in the context of motor rehabilitation. Recent studies indicate that motor training closely followed by sleep may result in better engagement of procedural (“how to”) memory consolidation processes in the elderly. Nevertheless, elderly individuals are mostly morning oriented and a common practice is to time rehabilitation programs to morning hours. Here, we tested whether the time-of-day wherein training is afforded (morning, 8-10:30 a.m., or evening, 6-9 p.m.) affects the long-term outcome of a multi-session motor practice program (10 sessions across 3-4 weeks) in healthy elderly participants. Twenty-nine (15 women) older adults (60-75 years) practiced an explicitly instructed five-element key-press sequence by repeatedly generating the sequence “as fast and accurately as possible.” The groups did not differ in terms of sleep habits and quality (1-week long actigraphy); all were morning-oriented individuals. All participants gained robustly from the intervention, shortening sequence tapping duration and retaining the gains (> 90%) at 1-month post-intervention, irrespective of the time-of-day of training. However, retesting at 7-months post-intervention showed that the attrition of the training induced gains was more pronounced in the morning trained group compared to the evening group (76 and 56.5% loss in sequence tapping time; 7/14 and 3/14 participants showed a > 5% decline in accuracy relative to end of training, respectively). Altogether, the results show that morning-oriented older adults effectively acquired skill in the performance of a sequence of finger movements, in both morning and evening practice sessions. However, evening training leads to a significant advantage, over morning training, in the long-term retention of the skill. Evening training should be considered an appropriate time window for motor skill learning in older adults, even in individuals with morning chronotype. The results are in line with the notion that motor training preceding a sleep interval may be better consolidated into long-term memory in the elderly, and thus result in lower forgetting rates.” As a personal observation, I have observed the same for myself for intellectual learning. I think I can better retain new information related to quantum physics or to molecular biology, for example, if I seek to digest it after supper.
Best time for wound healing
Limited research suggests that if are going to get a skin wound, the best time for that to happen from a healing perspective is during the day rather than at night. The 2017 publication Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing reports: “Fibroblasts are primary cellular protagonists of wound healing. They also exhibit circadian timekeeping, which imparts an approximately 24-hour rhythm to their biological function. We interrogated the functional consequences of the cell-autonomous clockwork in fibroblasts using a proteome-wide screen for rhythmically expressed proteins. We observed temporal coordination of actin regulators that drives cell-intrinsic rhythms in actin dynamics. In consequence, the cellular clock modulates the efficiency of actin-dependent processes such as cell migration and adhesion, which ultimately affect the efficacy of wound healing. Accordingly, skin wounds incurred during a mouse’s active phase exhibited increased fibroblast invasion in vivo and ex vivo, as well as in cultured fibroblasts and keratinocytes. Our experimental results correlate with the observation that the time of injury significantly affects healing after burns in humans, with daytime wounds healing ~60% faster than nighttime wounds. We suggest that circadian regulation of the cytoskeleton influences wound-healing efficacy from the cellular to the organismal scale.”
Note that this study is with mice as are many of the other circadian-impact studies, and that a significant proportion of the studies are with drosophila or other insects. The similarity of clock gene operation is so great, however, that conditionally, many of the results of these lower-species studies probably apply to us.
Best times to take anti-inflammatory dietary supplements
Research cited above suggest that inflammatory and wound healing processes are most active during daytimes, least active during dark hours of rest. The best approach for avoidance of inflammation-related damage as potentiated by daytime-active FLS genes, I infer, is therefore first thing in the morning. I take 4-Herb Synergy, Lion’s Mane and Turkey Tail mushroom extracts and a number of anti-inflammatory NF-kB inhibiting and Nrf2-promoting herbs extracts then. I also take smaller quantities of these substances just before going to bed to help stiffness and pain from emerging overnight. I believe these strategies are working for me.
Simple practical inputs to circadian clocks
- To help us get to sleep at night, No caffeine after noon, last meal of day at least 3 hours before bedtime, schedule heavy exercising in late morning or afternoon, avoid bright blue light and no fighting with relatives for 3 hours before bedtime, 3-5 mg of melatonin before bedtime. GABA supplements at bedtime might help too.
- To shake off grogginess in morning when getting up, expose yourself to bright natural daylight, using “daylight” artificial lights if necessary. Go outside for a while and move around If you can. Start day with at least moderate exercise, and a cup of coffee of course.
TRACKING SLEEP WITH WEARABLES
Several wrist, ring and other wearable devices on the market now allow for fairly extensive sleep and circadian pattern tracking, tracking that was formerly available only via staying overnight at sleep labs. I briefly characterize only two of these devices here, ones that I personally wear and use.
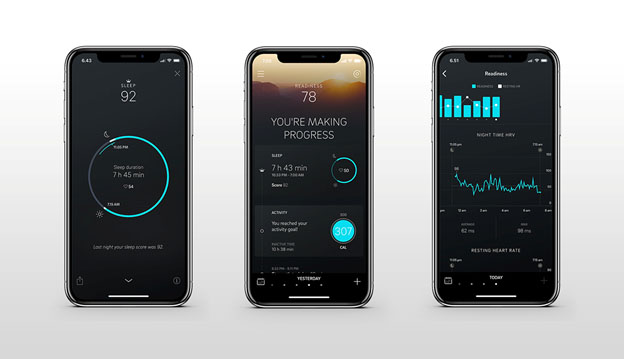
The Fitbit Charge 3 is a relatively low-cost multifunction watch-type activity tracker $125 to $150). It does a fairly good job at tracking steps, floors climbed swimming and various other exercises. It tracks total sleep, occurrences and lengths of sleep stages (light, deep and REM) during the night and pattern of resting heart rate. Just touch your device to see the time, your heart rate at the time, notifications, steps you have taken or other features. Summary daily data are available on an online or cell phone display. While accuracy of sleep measurements have been questioned in some reviews, I have found comparisons of one night with other nights to be sometimes valuable. A number of other useful parameters are displayed. Synchs automatically with my Android phone. Waterproof, having a clear screen, providing notifications and other smart-watch features, and having a 7 day battery life between recharging. Charging itself is very rapid, taking perhaps 40 minutes. I find the Charge 3 to be a highly reliable and practical device.
My Oura Ring looks like a wedding band, has no display of its own, but is purported to have significantly greater accuracy for measuring pulse and sleep events and even a version of heart rate variability. This is because pulse and circulation are less-masked by body tissues and more measurable at the base of a finger than on the wrist. The Oura Ring tracks almost all the same things the Fitbit Charge 3 does (measures body temperature in addition but not flights of steps climbed). However, my own experience and several independent reviews support Oura’s claims to enhanced accuracy when it comes to sleep and heart events. Below are a few of many screens available on the Oura app. Like the Fitbit Charge 3, the ring synchronizes to my Android phone via Bluetooth. The Android display for the ring provides greater detail related to sleep than does the display for the Fitbit. The Oura Ring currently goes for $299 for silver or black. I think it is worth the money for people interested in 24-7 monitoring their heart-rate, HRV or sleep parameters. If you just want to monitor your steps and exercise , the Fitbit is probably all you need. “Oura is more than just intelligent technology. It’s a philosophy to guide you to understand how your body responds to your activities, daily choices and rhythms.“
How do the Fitbit Charge 3 and Oura Ring data compare for the same night of sleep? They generally agree to within 80% or so. When in doubt I tend to trust the Oura Ring numbers. Downsides for the Oura Ring are that the battery must be recharged every 3 days and that takes a couple of hours. And it is usually a struggle to get the ring off past a bulge in my finger so it can be recharged. Finally, I sometime have trouble getting Bluetooth to synchronize between my phone and ring. That does not happen automatically as it does with the Fitbit device.
NEW FINDINGS ON OUR OLD FRIEND – MELATONIN
Melatonin is a hormone that is naturally expressed around bedtime to facilitate sleep. I have long used melatonin, 3-5 mg, to reset my body clock at bedtime and help assure a night of sound sleep. I started doing this back in the 60s and 70s when I frequently traveled to Europe or the Middle East for work, and found it helpful for quickly resetting my master clocks – in a day or two instead of a week or more required if I just let them reset themselves for a 5-10 hour time shift.
The 2018 publication New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation reports: “In mammals, a central circadian clock, located in the suprachiasmatic nuclei (SCN) of the hypothalamus, tunes the innate circadian physiological rhythms to the ambient 24 h light–dark cycle to invigorate and optimize the internal temporal order. The SCN‐activated, light‐inhibited production of melatonin conveys the message of darkness to the clock and induces night‐state physiological functions, for example, sleep/wake blood pressure and metabolism. Clinically meaningful effects of melatonin treatment have been demonstrated in placebo‐controlled trials in humans, particularly in disorders associated with diminished or misaligned melatonin rhythms, for example, circadian rhythm‐related sleep disorders, jet lag and shift work, insomnia in children with neurodevelopmental disorders, poor (non‐restorative) sleep quality, non‐dipping nocturnal blood pressure (nocturnal hypertension) and Alzheimer’s disease (AD). The diminished production of melatonin at the very early stages of AD, the role of melatonin in the restorative value of sleep (perceived sleep quality) and its sleep‐anticipating effects resulting in attenuated activation of certain brain networks are gaining a new perspective as the role of poor sleep quality in the build‐up of β amyloid, particularly in the precuneus, is unraveled. As a result of the recently discovered relationship between circadian clock, sleep and neurodegeneration, new prospects of using melatonin for early intervention, to promote healthy physical and mental ageing, are of prime interest in view of the emerging link to the etiology of Alzheimer’s disease.”
Endogenous production of melotonin is regulated by light incident on the retina. That is why clearing out a “foggy head” due to high presence of melatonin in mornings can be greatly assisted by exposure to bright daylight.
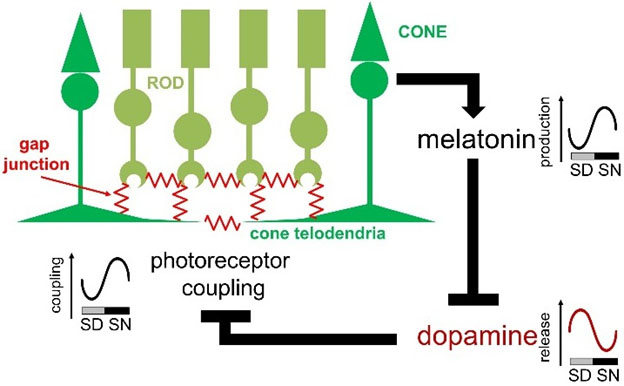
Image source: Ocular Clocks: Adapting Mechanisms for Eye Functions and Health “Overview of photoreceptor gap junction coupling and the melatonin/dopamine pathway. Gap junctions are located at photoreceptor terminals. Melatonin production is under the control of a clock within the photoreceptor layer and high at night. Melatonin suppresses dopamine release, and it is the nocturnal decrease in dopamine release and the subsequent decrease in activity of dopamine D2/4 receptors on photoreceptors that increases photoreceptor coupling. SD, subjective day; SN, subjective night.”
It is now 9:23PM, and time for me to publish this article before I start disrupting tonight’s circadian cycle. Although research in the field of chronobiology goes back to the 18th century, my feeling is that we still have very much to learn about this area, and we will discover more related practical health and longevity-promoting interventions. So, please stand by for more blogs related to this area.


Pingback: Unlocking Longevity - AGINGSCIENCES™ - Anti-Aging Firewalls™