By Vince Giuliano

INTRODUCTION
This blog entry is concerned with PHOTOBIOMODULATION, the first topic to be covered in a series of blog entries characterizing interventions for initiating natural processes for reverse aging.
The introduction to this series in provided in the previous blog entry PRACTICAL INTERVENTIONS FOR YOUNGING1.0 – Introduction to the series. It mentions the interventions we plan to cover in this blog series, identifies our criteria for selecting them, additional highly relevant interventions, what we expect to say about each intervention, and a list of the interventions I (Vince) am personally pursuing.
The concept of using light therapy for diseases is far from new. Colors were strongly associated with specific healing properties in ancient Egypt, China, India and Greece(ref). “In the 19th century, the chronic and progressive nature of this disease (Lupus Vulgaris) was particularly marked: it remained active for ten years, twenty years, or even longer and, proved resistant to all treatment until the breakthrough by Niels Ryberg Finsen using a form of “concentrated light radiation” now known as Photobiomodulation (PBM) which won him a Nobel Prize. Queen Alexandra of Great Britain, (1844–1925), consort to Edward the VII, as the inscription on the bronze statue of her at the London Hospital, notes, “Introduced to England the Finsen light cure for Lupus, and presented the first lamp to this hospital(Wikipedia)”. Later, around 2001 the theory and practice of PBM was greatly accelerated by the availability of appropriate medical-grade lasers, and starting around 2016 further accelerated by the availability of inexpensive LED devices that emit near-infrared and red light at therapeutic frequencies.
RED AND NEAR-INFRARED LIGHT PHOTOBIOMODULATION
A number of forms of electromagnetic radiation are known to produce positive health effects when doses are within hormetic ranges. We are concerned here particularly with red light in the 660 nm range and near infrared light in the 880 nm range. I (Vince) have long been aware of photobiomodulation (PBM) as a technique for achieving positive biological impacts. But it is not until recently, as part of my YOUNGING explorations, that I have seriously paid attention to it. I find myself blown away by the extent and depth of research related to bio modulation, the incredible array of health benefits it can produce, and the availability of inexpensive home equipment for PMB. “Photobiomodulation (PBM) therapy is a rapidly growing approach to stimulate healing, reduce pain, increase athletic performance, and improve general wellness(ref)”.
A. Known health and longevity benefits
Studies on the impacts of PBM have included rapid wound healing, tissue repair, treatment for scleroderma, Raynaud’s Syndrome, reduction and elimination of chronic pain, rejuvenation and proliferation of bone marrow, activation and renewal of adipose and dental stem cells, reduction of chronic inflammation, promotion of angiogenesis and neovascularization, reversal of age-related thymic involution, reversal of cell senescence markers, expansion and improvement of function of cells (like hematopoietic stem cells, embryonic stem cells, natural killer cells, islet-like cell clusters, neurons and glial cells), natural tissue repair, reversal of hair loss, protection of red blood cells, dialation of capillary vessels and associated cardiovascular and pulmonary benefits, enhancing angiogenesis in cases of injury recovery, triggering expression of nitric oxide in cases of spinal cord injury, treatment of ischemic stroke, treatment of acute spinal cord injury, reduction in auto-immune disease symptoms, treatment of fibromyalgia, treating eye injuries, enhancing cognitive function, creating immunotherapy against small cell lung cancer, reduction in edema, increasing blood flow to the brain, treating PTSD, treating restless leg syndrome, ophthalmic benefits like protection of the retinal barrier and reduction of age-related macular degeneration and retinal pathologies, treating all kinds of sports injuries, reduction of fibrosis, treatment of dermatitis, remedying SAD, enhancing HRV, increase endurance, ——
The list goes on and on and could fill an entire page. Far too many benefits to be believable? Snake oil? Too good to be true? Those are natural skeptical reactions. But the supportive research literature is there in plain sight for anyone who wants to pursue it. The National Library of Medicine database lists 13,417 publications for the term near-infrared therapy and 2002publications for the term photobiomodulation. The vast majority of these have been published in the lst seven years. A search in clinicaltrrialss.gov using the term near infrared therapy reveals 1,009 clinical trial studies and a search for photobiomodulation reveals 211 studies. (As I review this document prior to publication, I note that each of these numbers is higher than it was yesterday.)
The following images suggest applications of PBM

PBM applications Image source
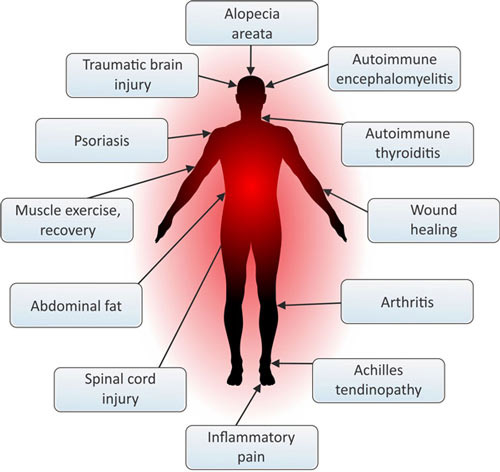
PBM applications Image source
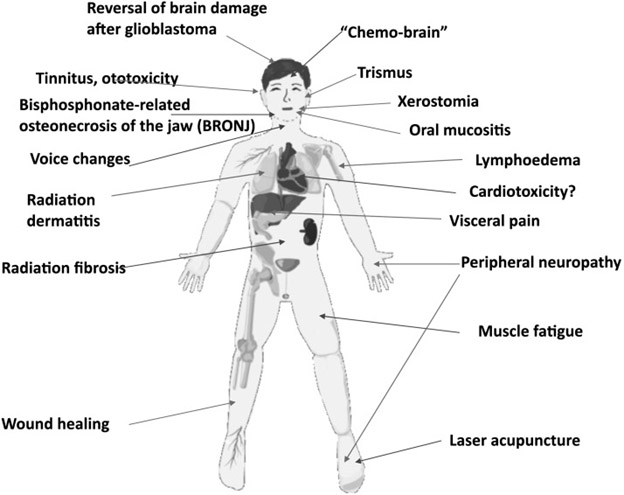
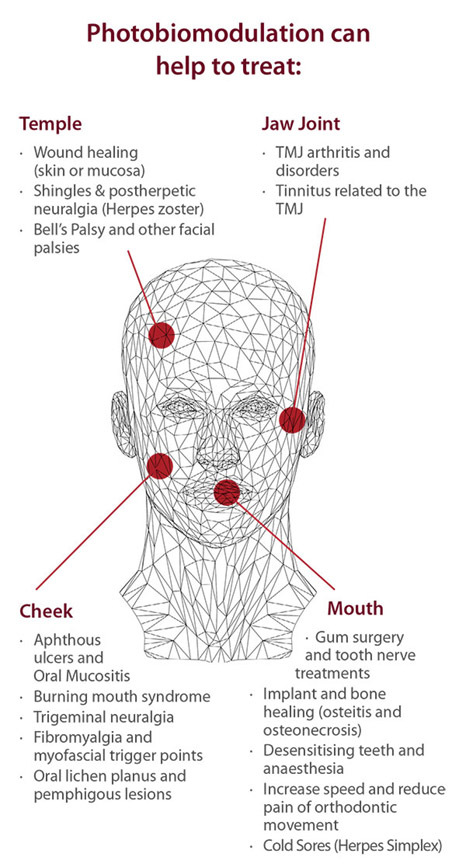
Applications of PBM Image Source
B. Literature citations supporting health and longevity benefits
Following is a small sample list of recent research publications beginning to show the range of PBM applicability. You may click to read any of interest.
- Photobiomodulation effect on the proliferation of adipose tissue mesenchymal stem cells.
- Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhBMP4-loaded hydrogel directs hard tissue bioengineering.
- Near Infrared Photoimmunotherapy for Cancer
- Near Infrared (NIR) Light Therapy of Eye Diseases: A Review
- Beneficial Effects of Near-Infrared Light Photobiomodulation in Linear Morphea: A Case Report.
- Infrared-mediated hyperthermia is effective in the treatment of scleroderma-associated Raynaud’s phenomenon
- Irradiation with Water-Filtered Infrared A plus Visible Light Improves Cutaneous Scleroderma Lesions in a Series of Cases
- Phototherapy in Scleroderma
- Photobiomodulation and Cancer: What Is the Truth?
- Photobiomodulation in Oral Medicine. 2019
- Photobiomodulation and Oral Mucositis: A Systematic Review.
- Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. 2021
- Photobiomodulation therapy as a high potential treatment modality for COVID-19. 2020
- Photobiomodulation Dose Parameters in Dentistry: A Systematic Review and Meta-Analysis.
- Photobiomodulation: Shining Light on COVID-19.
- Photobiomodulation via multiple-wavelength radiations.
- Photobiomodulation in human muscle tissue: an advantage in sports performance?
- Aging of lymphoid organs: Can photobiomodulation reverse age-associated thymic involution via stimulation of extrapineal melatonin synthesis and bone marrow stem cells?
- Photobiomodulation with 630 plus 810 nm wavelengths induce more in vitro cell viability of human adipose stem cells than human bone marrow-derived stem cells
- Red/Near Infrared Light Stimulates Release of an Endothelium Dependent Vasodilator and Rescues Vascular Dysfunction in a Diabetes Model
- Effects of photobiomodulation on mitochondria of brain, muscle, and C6 astroglioma cells
- Mitochondrial Dysfunction and Parkinson’s Disease—Near-Infrared Photobiomodulation as a Potential Therapeutic Strategy
- The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes
- Impact of photobiomodulation therapy on the morphological aspects of submandibular gland submitted to excretory duct ligation and hypothyroidism: an animal study.
- Accelerated Wound Healing Using a Novel Far-Infrared Ceramic Blanket.
- Combined Intense Pulsed Light and Low-Level Light Therapy for the Treatment of Dry Eye: A Retrospective Before-After Study with One-Year Follow-Up.
- The bioenergetics of COVID-19 immunopathology and the therapeutic potential of biophysical radiances.
- Quantum biology in low level light therapy: death of a dogma.
- Revisiting the Photon/Cell Interaction Mechanism in Low-Level Light Therapy.
- Light Effect on Water Viscosity: Implication for ATP Biosynthesis.
- Pre-conditioning with near infrared photobiomodulation reduces inflammatory cytokines and markers of oxidative stress in cochlear hair cells.
- Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts.
- Differential response of human dermal fibroblast subpopulations to visible and near-infrared light: Potential of photobiomodulation for addressing cutaneous conditions.
- Clinical and experimental applications of NIR-LED photobiomodulation.
- Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders.
- Clinical evaluation of single and repeated sessions of photobiomodulation with two different therapeutic wavelengths for reducing postoperative sequelae after impacted mandibular third molar surgery: a randomized, double-blind clinical study
- Impact of photobiomodulation therapy on the morphological aspects of submandibular gland submitted to excretory duct ligation and hypothyroidism: an animal study.
- Accelerated Wound Healing Using a Novel Far-Infrared Ceramic Blanket.
- Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain?
- Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy
- Combined Intense Pulsed Light and Low-Level Light Therapy for the Treatment of Dry Eye: A Retrospective Before-After Study with One-Year Follow-Up.
- Low-level light therapy potentiates NPe6-mediated photodynamic therapy in a human osteosarcoma cell line via increased ATP.
- Low-level light therapy of the eye and brain.
- Photobiomodulation as an antioxidant substitute in post-thawing trauma of human stem cells from the apical papilla.
- Effect of photobiomodulation in secondary intention gingival wound healing-a systematic review and meta-analysis.
- ESuccessful GaAlAs low-level laser therapy of self-inflicted thermal burns of the palate.
- Wavelength- and irradiance-dependent changes in intracellular nitric oxide level.
C. Biomolecular Pathways
Several biomolecular mechanisms have been proposed for explaining the biological activities of PBM and near infrared light. Some or most of these may work together synergistically. I review a few of the most familiar ones here, and then go on to review how quantum effects, unfamiliar for most biologists, may be key.
Conventional explanations
The most popular explanation revolves around a purported role of cytochrome c oxidase (COX) in mitochondria, as illustrated in this graphic.
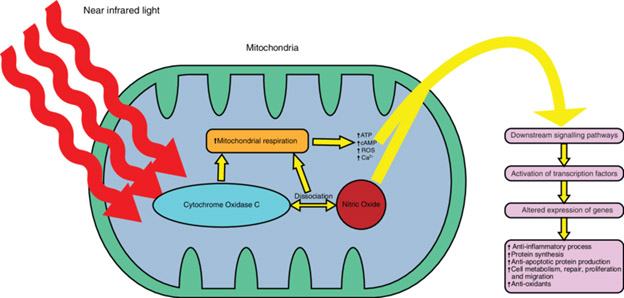
Image source “Schematic diagram of the mechanisms of photobiomodulation near infrared light within wavelengths (630-1000 nm) targets the mitochondrial enzyme cytochrome oxidase C resulting in (i) direct stimulation in mitochondrial respiration and (ii) dissociation of nitric oxide which indirectly increases mitochondrial respiration. These processes result in elevation of ATP, cAMP, reactive oxygen species and intracellular calcium which impact downstream signalling pathways that triggers increase in anti-inflammatory processes, protein synthesis, anti-apoptotic protein production, cellular repair/metabolism/proliferation/migration and antioxidants.”
This explanation has been seriously challenged, for example in the publication What Lies at the Heart of Photobiomodulation: Light, Cytochrome C Oxidase, and Nitric Oxide—Review of the Evidence
“Objective: The underlying mechanisms of photobiomodulation (PBM) remain elusive. The most attractive hypotheses revolve around the role of cytochrome c oxidase (CCO) and cellular energetics. — Background: No reliable demonstration of any PBM-related light-induced mechanistic effect on CCO has been reported. Studies on PBM have proven to be either nonreproducible, of questionable relevance, or involve wavelengths unlikely to be operative in vivo. The literature reveals very few demonstrable mechanistic light effects of any sort on CCO. Nitric oxide (NO) is involved in a number of the reported light effects on CCO. NO inhibits CCO at high reductive pressures by binding to the heme a3 moiety. This complex is white light labile. – Methods: The reported photolability of the heme–NO complex seems to be a prime target for PBM studies, as removal of inhibiting NO from the active site of CCO could restore normal activity to inhibited CCO. Another aspect of CCO–NO chemistry has been revealed that shows intriguing possibilities. — Results: A novel nitrite reductase activity of solubilized mitochondria has been demonstrated attributable to CCO. NO production was optimal under hypoxic conditions. It was also found that 590 nm irradiation increased NO production by enhancing NO release. The presence of cellular NO has usually been considered metabolically detrimental, but current thinking has expanded the importance and the physiological roles of NO. Evidence shows that NO production is likely to play a role in cardioprotection and defenses against hypoxic damage. — Conclusions: Studies combining PBM and hypoxia also point to a connection between light irradiation, hypoxia protection, and NO production. This leads the authors to the possibility that the intrinsic nature of PBM involves the production of NO. The combination of CCO and hemoglobin/myoglobin NO production with photorelease of NO may constitute the heart of PBM.”
Another of the conventional explanations for how PBM works via mitochondria is illustrated in this diagram.
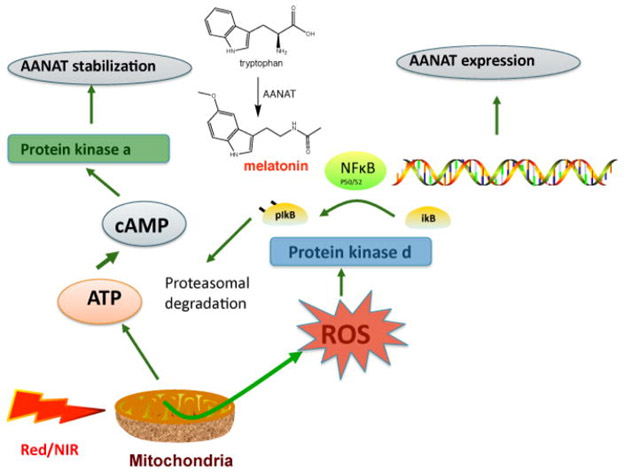
“The mitochondrial electron transport chain has been shown to be photosensitive to red and near-infrared (NIR) light. — mitochondrial photostimulation has been shown to increase ATP production and cause transient increases in reactive oxygen species (ROS). In some cells, this process appears to participate in reduction/oxidation (redox) signaling. Redox mechanisms are known to be involved in cellular homeostasis and proliferative control(ref: Low-Intensity Light Therapy: Exploring the Role of Redox Mechanisms).” The production and expression of mitochondrial ROS involve extremely complex biomolecular mechanisms and depend on many factors. For those who care to pursue it, this topic is treated in detail in the publication, Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Among the factors determining mitochondrial ROS production is mitochondrial membrane potential state, Δψm in the following diagram. This is possibly responsible for the major action of PBM on mtROS.
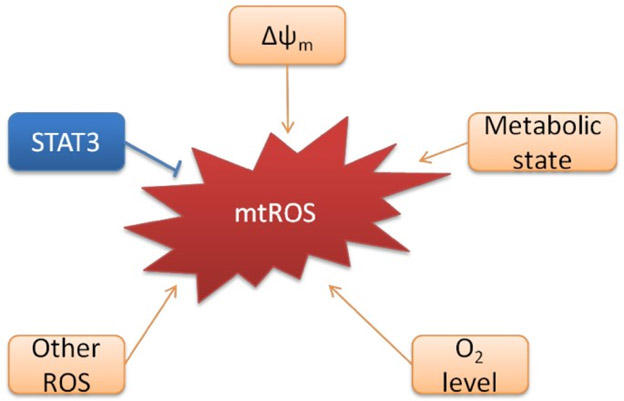
Image source “Regulation of mtROS production.
“A number of factors including mitochondrial membrane potential (Δψm), metabolic state of mitochondrial, O2 concentration regulate the production of mtROS. Non-mitochondrial generated ROS can also augment mtROS production, a process known as “ROS-induced ROS”. Meanwhile, transcription factor STAT3 has recently been found to suppress mtROS production independent of its nuclear factor activity.), metabolic state of mitochondrial, O2 concentration regulate the production of mtROS. Non-mitochondrial generated ROS can also augment mtROS production, a process known as “ROS-induced ROS”. Meanwhile, transcription factor STAT3 has recently been found to suppress mtROS production independent of its nuclear factor activity.”
Evidence for need for an alternative explanation is set forward in the 1919 publication Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light—it is mitochondrial bound water: the principles of low-level light therapy. “According to the mainstream theory the root cause for mitochondrial ATP upregulation in response to irradiation of cells with red-to-near infrared (R-NIR) light is the absorption of R-NIR photons by cytochrome c oxidase (CCO). Here, I show that this theory is inadequate for an explanation of the experimental results obtained in LLLT. Putting a model on a wrong concept will automatically limit its predictive capability, leading to results in disagreement with observation—as it happens today in LLLT. Excellent clinical and experimental results are contrasted by an underlying concept, i.e., the light/cell-interaction mechanism assumed in the majority of reports, which originates from a handful of articles showing incorrect data. Instead, a new theoretical basis for LLLT is presented. The resulting model describes experiments correctly and allows us to predict their outcome, thereby safeguarding future progress in LLLT.”
Another, probably more accurate explanation which depends on the role of nano-scale water layers in mitochondria is laid out in the 2017 publication Aging Is a Sticky Business. “Objective: The objective of this work is to put forward a mechanism by which low-level light [red-to near infrared (NIR) laser or light emitting diodes (LED)] is instrumental in the process of accelerating the healing of wounds. — Background data: Interaction modalities of low-level light with oxidatively stressed cells and tissues are the focus of intense research efforts. Several models of the light/cell-interaction mechanism have been proposed. In the most popular model, cytochrome c oxidase is believed to play the role of the principal acceptor for red-to NIR photons. — Methods: Using as an illustrative example the successful LED treatment of an edematous limb ulcer, the results of recent in vitro tests and complementary laboratory experiments are presented and discussed. — Results: The most plausible mechanism of biostimulatory effect of red-to NIR light consists of its impact on the nanoscopic interfacial water layers in mitochondria and the extracellular matrix (ECM) where mitochondrial reactive oxygen species (ROS) induce an increase in the viscosity of the water layers bound to the predominantly hydrophilic surfaces in the intramitochondrial space as well as the ECM, where the process progressively propagates with age. The biostimulatory effect of red-to NIR light consists of counteracting the ROS-induced elevation of interfacial water viscosities, thereby instantly restoring the normal mitochondrial function, including the synthesis of adenosine triphosphate (ATP) by the rotary motor (ATP synthase). — Conclusions: An understanding of the mechanism of interaction of red-to NIR light with mitochondria, cells, and tissues safeguards progress in the field of low-level light therapy (LLLT) and puts us in the position to design better therapies.”
Quantum theory explanation
The conventional explanations of the physical mechanisms through which PBM generates its health effects share a crucial flaw. They are only in terms of molecular and ordinary biology, whereas the interaction of radiation and matter is governed by a more-fundamental and absolutely validated body of science, quantum physics. This is possibly because very few scientists versed in molecular biology are familiar with quantum theory and its strange effects. Imagine how lame explanations of astronomy would be by scientists who ignore observations by telescopes, or how lame discussions of infectious diseases would be by scientists who ignore microscopic evidence. So when biologists who do not understand the quantum physics involved in the interaction of red/near infrared light and human tissues try to explain what happens that is really a quantum effect, what can we expect?
The 2020 publication Quantum biology in low level light therapy: death of a dogma amplifies on this theme and points out how the quantum effect of electron tunneling may play an important role when red/near infrared light is shined on human tissues. It picks up on the idea of PBM changing the viscosity of nano-scale water layers in mitochondria, and goes on to describe the quantum mechanism. . “Background: It is shown that despite exponential increase in the number of clinically exciting results in low level light therapy (LLLT), — . Progress in LLLT is currently realized by a trial and error process, as opposed to a systematic approach based on a valid photon-cell interaction model. — Methods: The strategy to overcome the current problem consists in a comprehensive analysis of the theoretical foundation of LLLT, and if necessary, by introducing new interaction models and checking their validity on the basis of the two pillars of scientific advance (I) agreement with experiment and (II) predictive capability. The list of references used in this work, does contain a representative part of what has been done in the photon-cell interaction theory in recent years, considered as ascertained by the scientific community. — Results: Despite the immense literature on the involvement of cytochrome c oxidase (COX) in LLLT, the assumption that COX is the main mitochondrial photoacceptor for R-NIR photons no longer can be counted as part of the theoretical framework proper, at least not after we have addressed the misleading points in the literature. Here, we report the discovery of a coupled system in mitochondria whose working principle corresponds to that of field-effect transistor (FET). The functional interplay of cytochrome c (emitter) and COX (drain) with a nanoscopic interfacial water layer (gate) between the two enzymes forms a biological FET in which the gate is controlled by R-NIR photons. By reducing the viscosity of the nanoscopic interfacial water layers within and around the mitochondrial rotary motor in oxidatively stressed cells R-NIR light promotes the synthesis of extra adenosine triphosphate (ATP). — Conclusions: Based on the results of our own work and a review of the published literature, we present the effect of R-NIR photons on nanoscopic interfacial water layers in mitochondria and cells as a novel understanding of the biomedical effects R-NIR light. The novel paradigm is in radical contrast to the theory that COX is the main absorber for R-NIR photons and responsible for the increase in ATP synthesis, a dogma propagated for more than 20 years.”
According to this publication, Quantum electron tunneling may be involved in what amounts to near-infrared light being the controlling element in a field effect transistor effect which governs the efficacy of the electron transport chain in affected mitochondria.
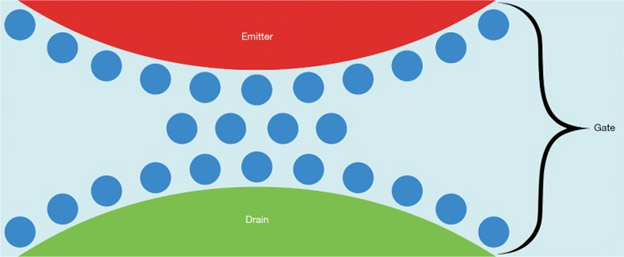
“Principle of the biological field-effect transistor (FET): The tunneling of electrons (not shown in the image) across the gate comprising the nanoscopic interfacial water layer formed by three monolayers of H2O (blue), confined between the enzymes cytochrome c acting as emitter (red) and cytochrome c oxidase (COX) acting as drain (green), is controlled by R-NIR photons. The expectation that exposure of the enzyme complex to biostimulatory intensities of R-NIR light induces an instant drop in the viscosity of the nanoscopic interfacial water layer confined in the space between emitter and drain, complemented by a spatial separation (volume expansion) is based on the results described in (44,49), respectively. The working principle of the FET can be understood on the basis of Eq. [1] and [2]. Image inspired by (48).”
In other words, in human tissues, levels of near infrared light level may control the flow of electrons between cytochrome c and cytochrome C oxidase, a key process in step IV of the mitochondrial electron transfer chain A powerful amplification effect. Far out science fiction? Actually very mundane. All modern electronic devices depend crucially on the same electron tunneling and FET process. Without them there would be no computers, cell phones, internet or TV, or pubmed for this blog you are reading for that matter. Engineers got used to this 50 years ago. Biologists, it is time for you to get on board to the underlying nature of reality. Read the document and the ones it cites for evidence.
D. How PBM activates JDJM3
As discussed above, PBM near-inrared and near red light perturbs mitochondrial functioning as well as generates excess mtROS, having a particular effect it is thought on Complex IV of the mitochondrial electron transfer chain. The 2019 publication Two Conserved Histone Demethylases Regulate Mitochondrial Stress-Induced Longevity describes the links between such mitochondrial perturbations and JDJM3 expression. “We identify the conserved histone lysine demethylases jmjd-1.2/PHF8 and jmjd-3.1/JMJD3 as positive regulators of lifespan in response to mitochondrial dysfunction across species. Reduction-of-function of the demethylases potently suppresses longevity and UPRmt induction while gain-of-function is sufficient to extend lifespan in an UPRmt-dependent manner. — These findings illustrate an evolutionary conserved epigenetic mechanism that determines the rate of aging downstream of mitochondrial perturbations.” The effect seems tp be mediated by the mitochondrial unfloded protein response (mtUPR). “We demonstrate that both jmjd-1.2 and jmjd-3.1 are necessary and sufficient for activation of the UPRmt in C. elegans. Moreover, our experiments identify jmjd-1.2 and jmjd-3.1 as positive regulators of a longevity response that genetically requires UPRmt signaling. Using transcriptome analysis, we demonstrate that jmjd-1.2 and jmjd-3.1 coordinate the transcriptional response to mitochondrial stress. Furthermore, using a systems genetics approach, we find that the mammalian orthologs exhibit positive genetic correlations with UPRmt core components in the BXD mouse genetic reference population (Andreux et al., 2012; Wu et al., 2014). Together, these data reveal a conserved epigenetic mechanism that determines longevity and stress signaling in response to mitochondrial dysfunction.”
Physical effects of heat
Another important physical process associated with red/near infrared radiation is which in terms leads to microvascular dilation associated with the expression of nitric oxide (NO) and the generation of elevated temperatures in the outer layers of the tissues irradiated, This can mean that growth and health factors generated in cells as a result of the radiation that can be released into the blood stream – say via being packaged in exosomes – can more effectively be carried by the circulatory system to more-distant organs.
E. Technology for PBM, red and near infrared light therapy
In the early recent history of PMB, starting in the 1960s the light sources used consisted mainly of lasers and the concept of low-level light therapy emerged. Starting about 2015, LED-based devices started to be used. Recent consumer-level therapy devices may embody arrays of hundreds of LEDs. The 2018 publication Photobiomodulation: lasers vs. light emitting diodes? reports: “Photobiomodulation (PBM) is a treatment method based on research findings showing that irradiation with certain wavelengths of red or near-infrared light has been shown to produce a range of physiological effects in cells, tissues, animals and humans. Scientific research into PBM was initially started in the late 1960s by utilizing the newly invented (1960) lasers, and the therapy rapidly became known as “low-level laser therapy”. It was mainly used for wound healing and reduction of pain and inflammation. Despite other light sources being available during the first 40 years of PBM research, lasers remained by far the most commonly employed device, and in fact, some authors insisted that lasers were essential to the therapeutic benefit. Collimated, coherent, highly monochromatic beams with the possibility of high power densities were considered preferable. However in recent years, non-coherent light sources such as light-emitting diodes (LEDs) and broad-band lamps have become common. Advantages of LEDs include no laser safety considerations, ease of home use, ability to irradiate a large area of tissue at once, possibility of wearable devices, and much lower cost per mW. LED photobiomodulation is here to stay.”
There is a wide variety of consumer-accessible LED PMB devices now available, These exist in a number of form factors, ranging from mini-flashlight sized devices to devices with arrays of LCDs to devices that fit in body orifices to panels, blankets or saunas that irradiate the entire body. To get an idea of devices marketed to consumers, you can start by checking on amazon,com for red light therapy lamps and near infrared therapy devices.
To start, here are the ones I have at home and have been using recently,
 Bulbs 54 watt– These with 18 LEDs. 9pcs 660nm deep red leds 9pcs 850nm + A From $25 to $40 at amazon.com. I (Vince)have a couple of these. I find their use relatively inconvenient because it is awkward to position them on some area of my body in a way that I can benefit from a therapy session while doing something else. The bulbs are so heavy that if put in a gooseneck table lamp they will bend it over so it shines straight downward or causes the entire lamp to fall over. But they [rovide a very low cost way to get started
Bulbs 54 watt– These with 18 LEDs. 9pcs 660nm deep red leds 9pcs 850nm + A From $25 to $40 at amazon.com. I (Vince)have a couple of these. I find their use relatively inconvenient because it is awkward to position them on some area of my body in a way that I can benefit from a therapy session while doing something else. The bulbs are so heavy that if put in a gooseneck table lamp they will bend it over so it shines straight downward or causes the entire lamp to fall over. But they [rovide a very low cost way to get started
 Smaller semi-flexible LED pads “Gospel’s” Equine & Canine Medium Light Therapy Pad 9-1/2″ X 12″ (30.5cm X 24.1cm) and has 132 LEDs! 60 visible red (660nm) and 72 near-infrared (850nm). Although marketed for animial use, they appear to work well for humans. I have found this pad useful for medium-small body parts like a hand or knee $424. Made in the US.
Smaller semi-flexible LED pads “Gospel’s” Equine & Canine Medium Light Therapy Pad 9-1/2″ X 12″ (30.5cm X 24.1cm) and has 132 LEDs! 60 visible red (660nm) and 72 near-infrared (850nm). Although marketed for animial use, they appear to work well for humans. I have found this pad useful for medium-small body parts like a hand or knee $424. Made in the US.

 Wraparound therapy sash DGYA) RLT-s21 -WT More area coverage than the smaller pad and physically flexible, like a heavy cloth. You can wrap it around your waist or an arm or leg and read, watch TV or work on your computer or rest during a 20 minute therapy session.
Wraparound therapy sash DGYA) RLT-s21 -WT More area coverage than the smaller pad and physically flexible, like a heavy cloth. You can wrap it around your waist or an arm or leg and read, watch TV or work on your computer or rest during a 20 minute therapy session.
After using this device daily for two weeks, I can declare it has reduced my lower back pain and discomfort by AT LEAST80%. Cost on Amazon,com $180 – $200
Here are some of the other device form factors
 Mini-flashlight, Helmet, Panel light
Mini-flashlight, Helmet, Panel light 



 Hat, Face mask, Hand enclosure
Hat, Face mask, Hand enclosure

 Foot LED device, joint pad, “full-body” panels
Foot LED device, joint pad, “full-body” panels

 Infrared sauna blanket Intranasal therapy light
Infrared sauna blanket Intranasal therapy light


 Infrared therapy saunas
Infrared therapy saunas
 Whole body PBM laser light pod
Whole body PBM laser light pod
A recent trend is that all over the country is that some local tanning salons are starting to acquire whole- body PBM treatment devices and offer PBM therapy. For randomly selected example see the web pages for Sun City Tanning in Pembroke MA, Superior Tanning in Kingston RI, and Sun Haven Tanning in Billings Montana. I find this interesting because of the very real benefits of PBM vs the mixed health benefits of UV for tanning, which can be of cosmetic value but can include promotion and inductions of skin cancers.
F. Limitations of current knowledge
In general, the field of PMB therapy has been driven much more by widespread practice and the delivery of positive health effects than by theory. Relatively little attention has been given to understanding of underlying mechanisms. “It works!” and that has led to widespread commercialization and popular use. So the weakest areas of understanding about it are 1. The fundamental physical and biological mechanisms through which it works, which I have focused on here,,and 2. Validated treatment protocols and dosage parameters for various disease and pathological conditions: What are the best frequencies of light to be used? Where should the light be shined? With what intensity? For how big an area? For how long? Repeated how often? And for how many times?
For most treatment purposes we simply don’t know the best answers to these treatment-related questions. Bur we also don’t know how big a problemef that is. The research and experience suggests that exactitude of these parameters may not be that critical. That is, therapy may be successful for a wide variety of conditions using standard default values. Manufacturers of most of the PBM devices mentioned above suggest using default values. For example, a medium sized LED therapy pad such as my Equine unit described above is applied directly to the skin; it enables therapy coverage of about 100 square inches, offers the same dose of red and near-infrad light at the same frequencies to whatever skin area it is applied to, and times itself out in 15 minutes


Pingback: PRACTICAL INTERVENTIONS FOR YOUNGING1.0 – Introduction to the series - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: PRACTICAL INTERVENTIONS FOR YOUNGING1.0 – Part 2: Electromagnetic health stimulation approaches and devices - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™