By James Watson, with contributions and editing by Vince Giuliano
We promised a major series of blog entries related to inflammation back in May 2016. We said we would do that through narrating a number of inter-related stories many of which seeming to have nothing to do with each other but which all come into relationship with each other at the end. This is Part 2 of that series. Part 1, already published, is the same as Part 5 of the NAD world. That blog entry is concerned with The pro-inflammatory effects of eNAMPT (extracellular NAMPT, nicotinamide phosphoribosyltransferase). This present blog entry takes a different approach, relating 1) the “master” pathway network of inflammation (NF-kB) to two other pathway networks clearly implicated in aging and disease processes 2) Genomic Instability (p53), and 3) Oxidative stress (Nrf2). Part 3 if this series is concerned with resolving inflammation, and the important roles of certain mediators, namely resolvins, protectins, maresins and lipoxins. These are found in fish oil! Part 4 of the inflammation series is concerned with an amazingly effective new approach for averting the biggest killers of older people, atherosclerosis and heart disease – that being inhibition of the PCSK9 protein. Other blog entries in this series will be forthcoming.
First, some general comments about inflammation and its relevance.
SECTION A – ABOUT CHRONIC INFLAMMATION
Background Section by Vince Giuliano
Inflammation is an important natural biological defensive response to many threatening agents such as pathological microorganisms, parasites, fungi, viruses, toxic compounds and pollutants. It is also a body response to of numerous deleterious health conditions including infections, wounding, and disease processes. Inflammation is a critical mechanism of the body’s innate immunity system, which seeks to eliminate the cause of irritation or injury, clear dead and necrotic cells, and is an essential initial stage of healing injured tissues. Inflammation is also induced by reactions of the immune system (hypersensitivity) in the body, which causes a disruption of tissue homeostasis.
So, inflammation is a very good thing. We could not be alive without it. Chronic inflammation, however, can be quite a different matter. In healthy circumstances, the inflammation response vanishes through a normal sequence of healing processes when no longer needed, after a few hours or a few days. However, in the presence of several disease processes and in the presence of aging, the inflammatory process may not progress through the final healing stages and the inflammation may become chronic. Chronic inflammation also results when the body’s defense system inappropriately triggers inflammation against its own cells. Chronic inflammation is both a causal factor and a consequence of most chronic diseases of aging, including rheumatoid arthritis, asthma, atherosclerosis, Alzheimer’s disease and cancer.
Chronic inflammation, sometimes referred to as constitutive inflammation, other times as inflammaging, can persist over an extended period of weeks to months and even years. It is often associated with the presence of macrophages and lymphocytes, fibrosis, vascular proliferation, and tissue destruction. Moreover, chronic inflammation plays critical roles in many disease processes including cancers, dementias, diabetes, pulmonary diseases, cardiovascular diseases, atherosclorsis, sarcopenia, and anaemia. Chronic inflammation occurs in the case of incurable autoimmune diseases such as arthritis, lupus, scleroderma, asthma and chronic obstructive pulmonary disease (COPD).
The biological mechanisms of chronic inflammation can be very complex, Nuclear factor- B (NF-κB) is activated by more than 200 different stimuli has for good reason been thought of as the master activator of inflammation. It is a central topic in this blog entry. For example, during inflammation immune system macrophage cells could be activated by Toll-like receptors (TLRs), through the recognition of a pathogen endotoxin such as lipopolysaccharide (LPS). This event initiates a signaling pathway that releases NF-κB into the cell nucleus, activating genes associated with the transcription of proteins related to the inflammatory process, such as iNOS, responsible for NO synthesis, COXs, which synthetize prostaglandins, and cytokines like IL-6. The generation of ROS is also triggered by the TLR signaling pathway.
Among the highly technical topics related to chronic inflammation and its consequences are Activating protein-1 (AP-1), AGEs, RAGE receptor, PAMPs, DAMPs, RNS, leukotrienes, LOX, prostaglandins, COX1, COX2, Resolvins, Protectins, Maresins, the NLRP3 inflammasome, lipoxins, Ca++ induced inflammation, pyroptosis, cellular senescence-induced inflammation, roles of inflammation in aging, potassium efflux out of a cell, mitochondrial ROS, translocation of NLRP3 to the mitochondria, cytosolic release of mitochondrial DNA, cardiolipin release, release of lysosomal cathepsin D into the cytosol, extracellular LPS “priming” of NLRP3, amyloid-beta “triggering” of NLRP3 via TLR4, ATP, and pore-forming toxins. We expect to touch on most of these in this blog series on inflammation.
Inflammation and aging
Since chronic inflammation plays central roles in numerous deleterious health processes and in aging, it is often referred to as “inflammaging” and is the subject of much ongoing research. From the 2014 publication Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases: “Human aging is characterized by a chronic, low-grade inflammation, and this phenomenon has been termed as “inflammaging.” Inflammaging is a highly significant risk factor for both morbidity and mortality in the elderly people, as most if not all age-related diseases share an inflammatory pathogenesis. Nevertheless, the precise etiology of inflammaging and its potential causal role in contributing to adverse health outcomes remain largely unknown. The identification of pathways that control age-related inflammation across multiple systems is therefore important in order to understand whether treatments that modulate inflammaging may be beneficial in old people.”
The 2016 publication Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity points out the central linkages between inflammation and aging. “Longevity and aging are two sides of the same coin, as they both derive from the interaction between genetic and environmental factors. Aging is a complex, dynamic biological process characterized by continuous remodeling. One of the most recent theories on aging focuses on immune response, and takes into consideration the activation of subclinical, chronic low-grade inflammation which occurs with aging, named “inflammaging.” Long-lived people, especially centenarians, seem to cope with chronic subclinical inflammation through an anti-inflammatory response, called therefore “anti-inflammaging.” In the present review, we have focused our attention on the contrast between inflammaging and anti-inflammaging systems, by evaluating the role of cytokines and their impact on extreme longevity. Cytokines are the expression of a network involving genes, polymorphisms and environment, and are involved both in inflammation and anti-inflammation. We have described the role of IL-1, IL-2, IL-6, IL-12, IL-15, IL-18, IL-22, IL-23, TNF-α, IFN-γ as pro-inflammatory cytokines, of IL-1Ra, IL-4, IL-10, TGF-β1 as anti-inflammatory cytokines, and of lipoxin A4 and heat shock proteins as mediators of cytokines. We believe that if inflammaging is a key to understand aging, anti-inflammaging may be one of the secrets of longevity.” This is an opinion I (Vince) hold. In a later blog entry in this series, I will share some of the approaches to anti-inflammaging I have been personally and professionally pursing.
NF-κB – a key driver of aging
This blog entry centers around the role of the transcription factor NF-κB , long known to be a master regulator of constituitive inflammation. But, NF-κB expression is also correlated with and appears to drive many aspects of aging. From the 2007 publication Motif module map reveals enforcement of aging by continual NF-κB activity: ”Aging is characterized by specific alterations in gene expression, but their underlying mechanisms and functional consequences are not well understood. Here we develop a systematic approach to identify combinatorial cis-regulatory motifs that drive age-dependent gene expression across different tissues and organisms. Integrated analysis of 365 microarrays spanning nine tissue types predicted fourteen motifs as major regulators of age-dependent gene expression in human and mouse. The motif most strongly associated with aging was that of the transcription factor NF-κB. Inducible genetic blockade of NF-κB for 2 weeks in the epidermis of chronologically aged mice reverted the tissue characteristics and global gene expression programs to those of young mice. Age-specific NF-κB blockade and orthogonal cell cycle interventions revealed that NF-κB controls cell cycle exit and gene expression signature of aging in parallel but not sequential pathways. These results identify a conserved network of regulatory pathways underlying mammalian aging and show that NF-κB is continually required to enforce many features of aging in a tissue-specific manner.”
What is NF- kB?
The significance of NF- kB expression has been highlighted in my writings throughout the last ten years. To start, a bit of clarification on what NF-kB is. From my 2009 blog entry A further update on NF-kappaB: NF-kappaB is not a single molecular substance but is “a collective name for inducible dimeric transcription factors composed of members of the Rel family of DNA-binding proteins that recognize a common sequence motif”(ref). What these proteins share in common is a motif, e.g. a characteristic DNA binding sequence. In simple language NF-kappaB is a collection of proteins that can profoundly affect the transcription of DNA, that is the production of messenger RNA and the subsequent productions of proteins encoded by DNA. NF-κB can target over 200 human genes in different kinds of cells. It has positive roles in maintaining health and also can create disease conditions and accelerate aging.
The gene sequence motif most closely associated with aging is that of NF-KappaB. “NF-kappaB is found in essentially all cell types and is involved in activation of an exceptionally large number of genes in response to infections, inflammation, and other stressful situations requiring rapid reprogramming of gene expression(ref). It is a very rapidly-acting substance, a “first responder” to harmful cellular stimuli. NF-kappB tends to be plentiful in cells of older people.
Normally, NF-kB lives in the cytoplasm of cells where it is bound up and kept out of the nucleus by a family of substances called IkB (inhibitor of kappaB). When a harmful extracellular stimulus is perceived, the IkB inhibitor molecules are modified by a process called ubiquitination and destroyed by cellular processes known as proteolysis. The result is that the NF-kappaB is freed to translocate into the nucleus where it can bind to a variety of genes, activate them and produce a variety of impacts including vicious pro-inflammatory ones. These processes are in fact quite complex involving many proteins, adapter, promoter and co-activator factors.
The illustration below suggest that NF-κB is the “master regulator of aging”. This may be a little bit of hyperbole, but it is clearly a very important aspect of aging.
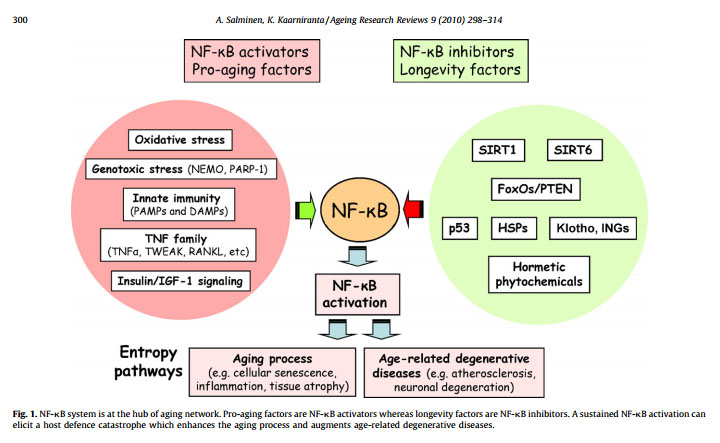
Reference: Motif module map reveals enforcement of aging by continual NF-κB activity
The following three diagrams illustrate why NF-κB is so important:
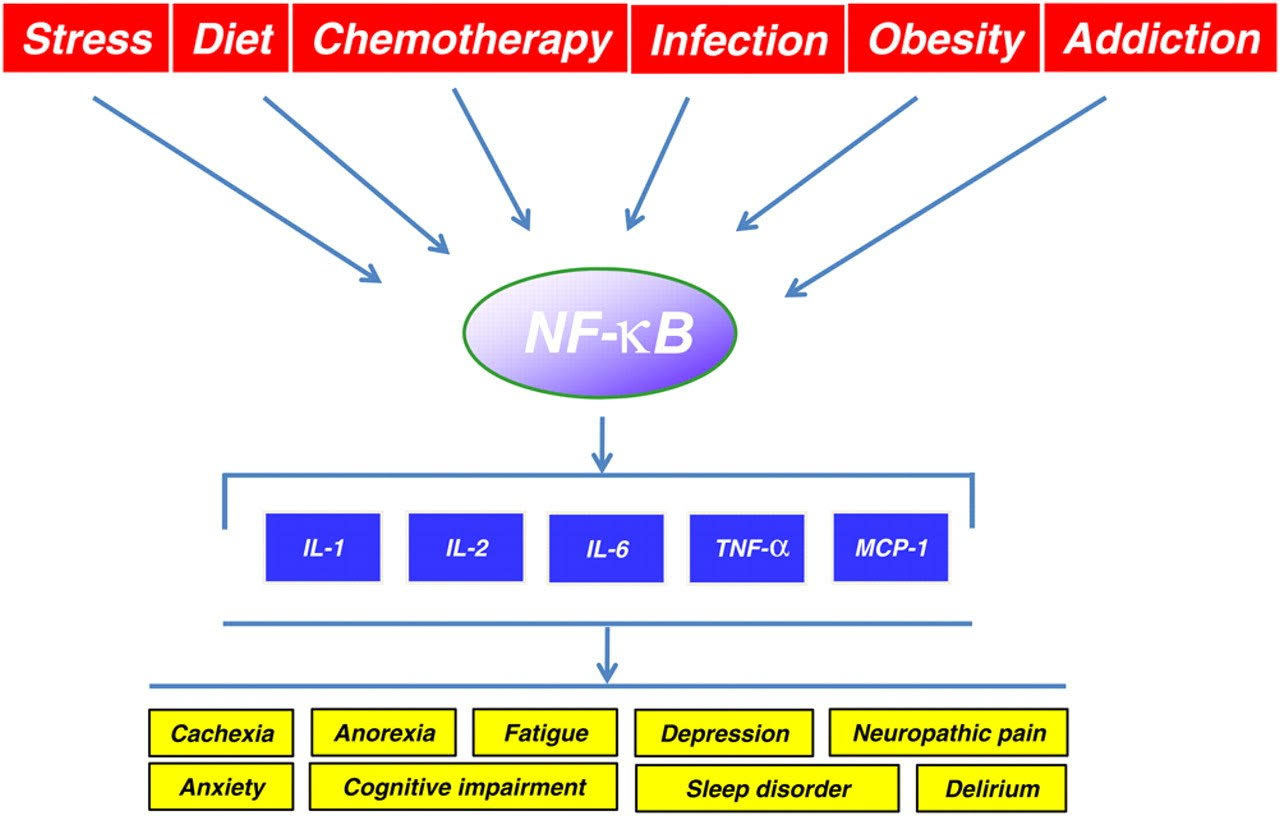
Image source Causes and results of NF-κB activation

Image source Diseases where chronic activation of NF-κB and inflammation are involved
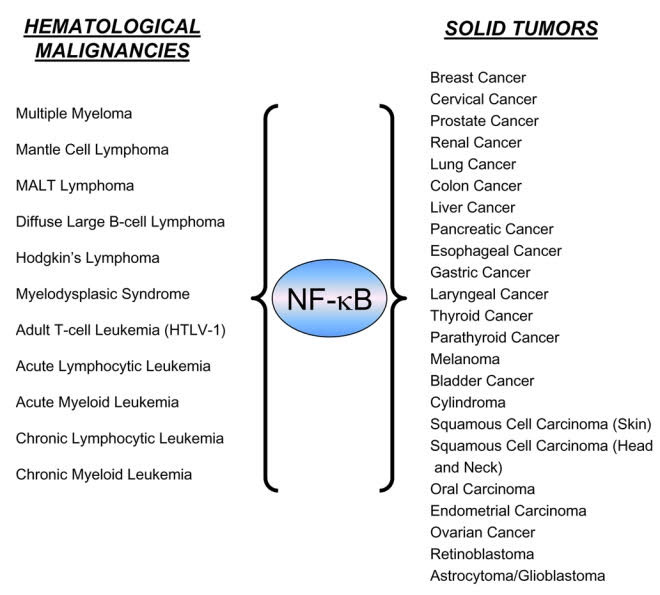
Image source
There is also a very strong link between negative regulation of NF-κB , inflammation and cancer, as outlined in the discussion below.
There are numerous inflammatory biological pathways and these are affected differentialy by different anti-inflamatory drugs or natural substances. Among these pathways are ones involving arachidonic acid, prostaglandins, leukotrienes and thromboxanes, microglial activation, cyclooxygenase COX enzymes, and nitric oxide synthase (iNOS), TNF-alpha, NLRP/NALP inflammasomes, P65, STAT3, and cytokines including IL-6 and IL-1beta. Proinflammatory cytokines can serve as chemoattractant for neutrophils and facilitate them sticking to the endothelial cells for migration. They also stimulate white cell phagocytosis and the production of inflammatory lipid prostaglandin E2 (PGE2).
In case of some medical conditions such as rheumatoid or osteo atheritis, inflammation can generate a great deal of persistent pain and discomfort. So substances and treatments for the control of chronic inflammation and associated pain are very important. In blog entries over recent years we have discussed this in terms of several different contexts, particularly in terms of histone acetylation/deacetylation and in terms of actions of certain plant polyphenols (ref)(ref)(ref)(ref).
SECTION B: THE TALE OF THREE STRESS SENSORS
By James P Watson
Introduction
I recently read a wonderful article written by authors from the “Department of Stress Biology” at Roswell Park Cancer Institute in Buffalo, NY. The title of their review article was “Inflammation and p53 – The Tale of Two Stressors“ (Andrei Gudkov, Katerina Gurova, and Elena Komarova).
This article triggered a burst of oxidative stress in my brain that has led me to write the following essay that is our second blog in the series of blogs on “Inflammation and Aging” (aka “Inflammaging”). Gudkov, Gurova, and Komarova pointed out that NF-kB and p53 pathways had significant “crosstalk” and that these two “Stress Sensors” affected each other in such a dramatic way that one pathway cannot really be analyzed without considering the other pathway. Then I started thinking about another “Stress Sensor” that many if you know very well which had similar interactions – the Nrf2 pathway. Vince was the one who taught me about the interactions between Nrf2/Keap1 and the NF-kB pathway. I have added that topic to the Tale of Three Stress Sensors essay. If these three pathways are depicted as a geometric triangle, then I suspected that there would also be crosstalk between p53 and the Nrf2/Keap1 pathways. When I explored this topic in a literature search, I was delighted to find out that some excellent research had been done, showing that p53 did affect Nrf2/Keap1 by suppressing the Nrf2-dependent transcription of ARE genes. Thus the geometric triangle of p53, NF-kB, and Nrf2 is complete, with clear interactions between all three pathways!
For this reason, I have titled this essay as The Tale of Three Stress Sensors, but it could also be called the Triangle of Interacting Stress Responses.
On inflammasomes
First I would like to talk about a caveat in my theory – the Inflammasome. It may be that this old evolutionary-stews response system should also be elaborated in this essay, but at this time I do not know enough about it to include it as a 4th pathway with the “Big Three” stress systems (p53, NF-kB, and Nrf2). So, I allude to inflammasomes several times but do not really treat them in this blog entry. That task is sufficiently important and complex as to merit devoting a subsequent blog entry to it in this Inflammation and Aging series.
To be clear, I am currently fascinated with inflammasomes – 500 million year old pathways that were just discovered in 2002. Inflammasomes are molecular-receptor complexes that self-assemble, and that are very “trigger happy” for the release of inflammatory cytokines upon multiple stimuli. Once activated, an inflammasome can tend to “lock in” chronic inflammation. From The Inflammasomes (2010) “Inflammasomes are molecular platforms activated upon cellular infection or stress that trigger the maturation of proinflammatory cytokines such as interleukin-1β to engage innate immune defenses. Strong associations between dysregulated inflammasome activity and human heritable and acquired inflammatory diseases highlight the importance this pathway in tailoring immune responses.”
The first Inflammasome was discovered by Jurf Tachopp at the University of Lausanne in 2002. This one was the NALP1 Inflammasome. Since then, many other unique Inflammasomes have been discovered, such as the NLRP3, NLRC4, and the AIM Inflammasomes. What all of them have in common is the activation of pro-inflammatory cytokine precursors (IL-1beta and IL-18 are the most important ones). Right now it is still not clear how important the role of IL-1beta is in aging, especially atherosclerosis. For this reason, two randomized, double-blinded, placebo-controlled studies have been started to try to down-regulate inflammation. One is using monoclonal antibodies against IL-1beta, called the CANTOS trial. The other is using low dose methotrexate (20 mg/week) and is called the CIRT trial. The CANTOS trial is being funded by Novartis and the CIRT trial is being funded by the National Heart Lung and Blood Institute (NHLBI). Are inflammasome structures the missing piece of the puzzle of “inflammaging?” At this point, I must say that we do not have enough proof for this hypothesis.
The Tale of Three Stress Sensors
Although the exact mechanism by which fasting turns off inflammation is not yet completely understood, it is clear is that the NF-κB transcription factor turns on inflammation. In fact, all of the genes for the molecular components of the Inflammasome structure (NLRP3, Caspace-11, and ASC) are “turned on” by NF-κB . As I was reading about all this, it was also clear to me that I really didn’t understand NF-κB . (especially all of its components, its upstream triggers, and its downstream effects. For this reason, I started reading and found three “Stress sensors” that I believe may be the 3 keys to understanding “inflammaging” – they are NF-κB , p53, and Nrf2.
True cause of “Inflammaging”
According to Gudkov, Gorova, and Komarova,the true cause of the low grade chronic inflammation seen with aging is due to Constitutive Activation of NF-kB. I had always suspected this, but I had not seen it in print. (See reference below). Here is an illustration of how this occurs:
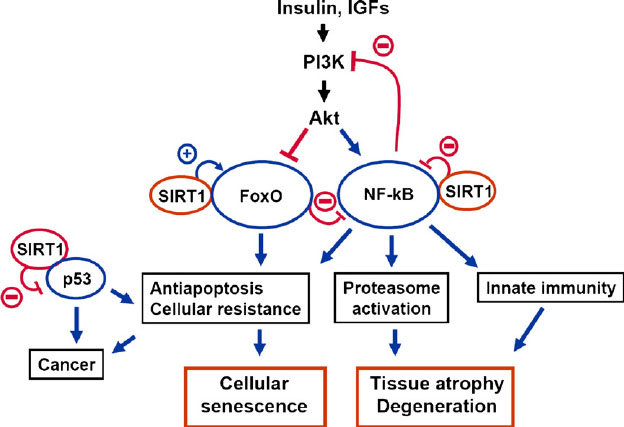
Reference and Image source: The signaling interplay between SIRT1, FoxO, and NF-kB in the regulation of cellular functions associated with the appearance of the mammalian aging phenotype
More recently, it has been discovered that constitutive STAT3 activation also activates inflammatory pathways and that this mechanism also does not require that the cell undergo cell cycle arrest. (See the 3rd illustration below). These “senescent-independent” inflammatory mechanisms make a lot of sense to me, since the constitutive activation of NF- B does not require that the cell have undergone senescence (NF-κB does contribute to senescence via activating the SASP genes in concert with STAT3, but NF-κB is NOT the cause of cellular senescence).
Whereas four factors explain cell senescence (p53, Rb, p21, p16INK4a), NF-κB can cause “inflammaging” in both senescent and non-senescent cells. Even in extreme old age, less than 15% of the body’s cells are senescent, but all cells exhibit molecular inflammaging with aging. What then explains all of the inflammation in the non senescent cells? The answer is NF-κB , but the story is much more complex than that! NF-κB is our best friend and our worse enemy, all bundled up into one transcription factor! How can this be? Well, if you really want to know, then read on!
Reference: 2011 Inflammation and p53 A Tale of Two Stresses “Numerous observations indicate a strong link between chronic inflammation and cancer. This link is supported by substantial experimental evidence indicating mutual negative regulation of NF-κB the major regulator of inflammation, and p53, the major tumor suppressor. This antagonistic relationship reflects the opposite principles of the physiological responses driven by these transcription factors, which act as sensors and mediators of intrinsic and extrinsic cell stresses, respectively. Constitutive activation of NF-κB , the underlying cause of chronic inflammation, is a common acquired characteristic of tumors. A variety of experimental methods have been used to demonstrate that constitutive activation of NF-κB reduces the tumor suppressor activity of p53, thereby creating permissive conditions for dominant oncogene-mediated transformation. Loss of p53 activity is also a characteristic of the majority of tumors and results in unleashed inflammatory responses due to loss of p53-mediated NF-κB suppression. O n the other hand, in natural or pharmacological situations of enforced p53 activation, NF-κB activity, inflammation, and immune responses are reduced, resulting in different pathologies. It is likely that the chronic inflammation that is commonly acquired in various tissues of older mammals leads to general suppression of p53 function, which would explain the increased risk of cancer observed in aging animals and humans. Although the molecular mechanisms underlying reciprocal negative regulation of p53 and NF-κB remain to be deciphered, this phenomenon has important implications for pharmacological prevention of cancer and aging and for new approaches to control inflammation.”
More illustrations of NF-κB and aging:
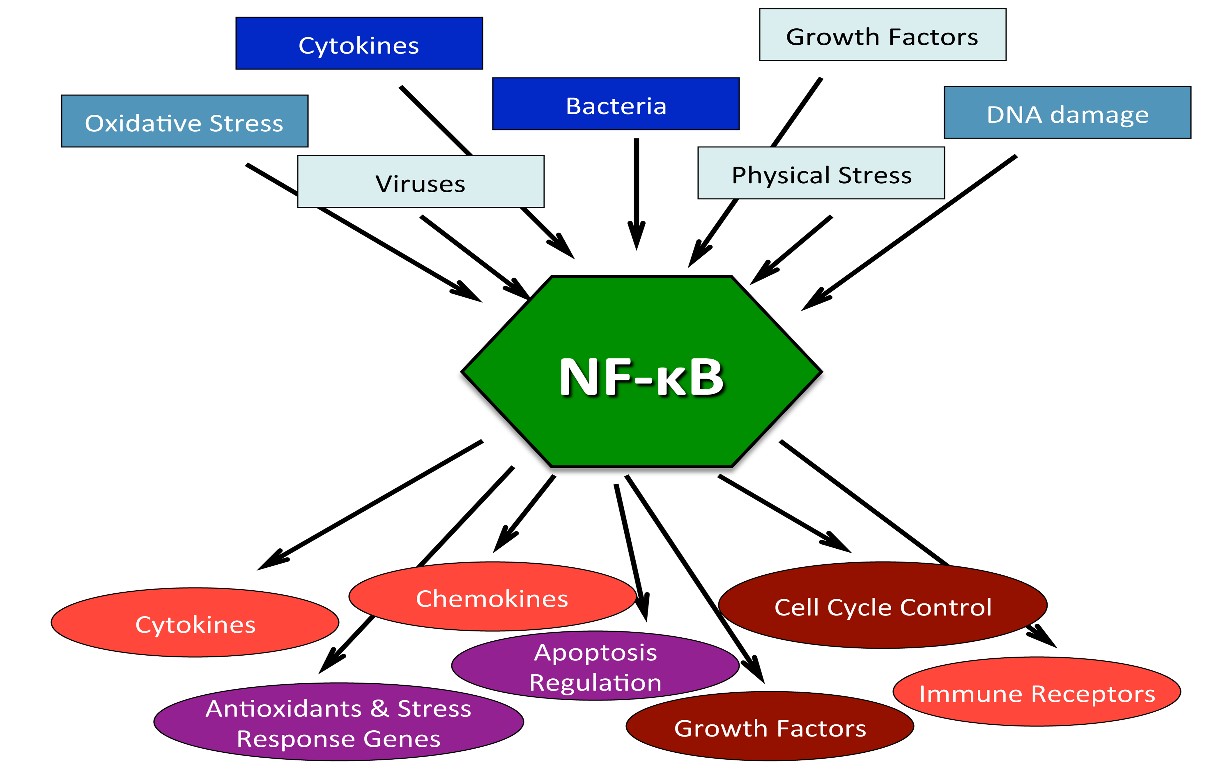
Reference: NF-κB in Aging and Disease “Stochastic damage to cellular macromolecules and organelles is thought to be a driving force behind aging and associated degenerative changes. However, stress response pathways activated by this damage may also contribute to aging. The IKK/NF-κB signaling pathway has been proposed to be one of the key mediators of aging. It is activated by genotoxic, oxidative, and inflammatory stresses and regulates expression of cytokines, growth factors, and genes that regulate apoptosis, cell cycle progression, cell senescence, and inflammation. Transcriptional activity of NF-κB is increased in a variety of tissues with aging and is associated with numerous age-related degenerative diseases including Alzheimer’s, diabetes and osteoporosis. In mouse models, inhibition of NF-κB leads to delayed onset of age-related symptoms and pathologies . In addition, NF-κB activation is linked with many of the known lifespan regulators including insulin/IGF-1, FOXO, SIRT, mTOR, and DNA damage. T hus NF-κB represents a possible therapeutic target for extending mammalian healthspan.” The diagram below shows how STAT3 and NF-κB work together to cause “inflammaging“. The diagram also shows that at least 4 cytokines can trigger these pathways (IL-6, TNF-a, IL-1A, and IL-1Beta).
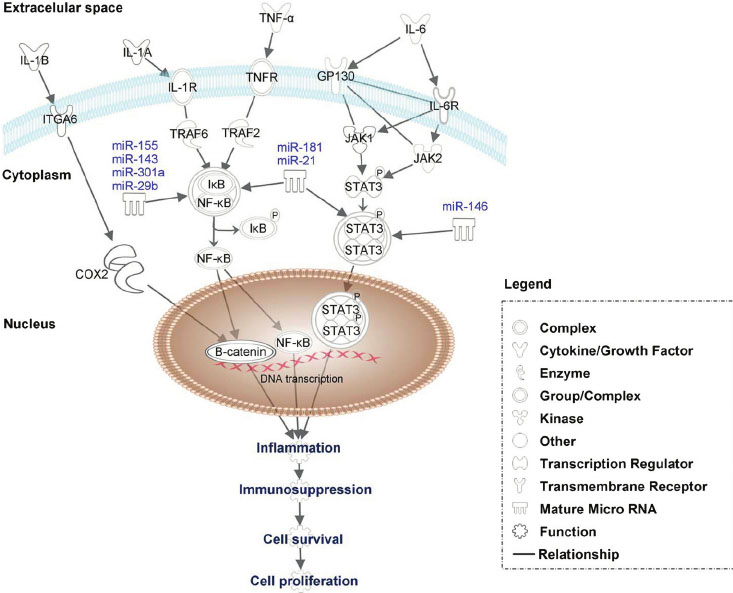
Image and legend source: 2013 Constitutive-activation-of-pro-oncogenic-inflammatory-pathways-including-STAT3 “Constitutive activation of pro-oncogenic inflammatory pathways including STAT3, NF-kB and B-catenin by proinflamatory factors such as IL-6, TNF- a , IL-1A and IL1-B. IL-6 activates via tyrosine kinases and cytokine receptors as JAK1 and JAK2 the STAT3, which after dimerization translocate to the nucleus, to directly activate genes involved in inflammation, immunosupression or cell proliferation. TNF- a and IL-1A modulate via TRAF6 and TRAF2 the IkB –NF-kB complex. IkB is released then from IkB –NF-kB complex by phosphorylation and NF- B translocate to the nucleus where activate specific set of genes involved in carcinogenesis. B-catenin can be activated by IL-1B proinflammatory factor via COX2. Moreover, the expression of STAT 3 and NF-kB can be together or separately modulated by specific miRNAs.”
Conclusion: there is ample evidence that the NF-kB pathway gets left in the “on position” as we age (also referred to as “constitutive activation”). Exactly why this pathway is “left on” could be due to a combination of cytokine signaling (IL-6, TNF-alpha, IL-1a, IL-1b), activation of the Insulin/IGF pathway, dysregulated microRNA, overactive mTOR signaling, or other reasons described below. Whatever the cause, this fact makes a strong case for the use of NF-κB inhibitors for the treatment of aging and age-related diseases. As we have discussed in previous blog entries, many strategies have been proposed to accomplish this including consumption of natural products (curcumin, EGCG, etc.) We also expect that the final blog entry in this inflammation series will in large part be devoted to these practical strategies.
NF-κB reduces p53 activity – the inflammation-cancer link
Now the molecular mechanisms of how inflammation causes cancer is coming to light! p53, of course is the master tumor suppressor, but when NF-κB is constitutively activated, the tumor suppressor activity of p53 is reduced! This explains why chronic inflammation and aging both are linked to an increase in cancer risk!
With any cell stressor such as excessive DNA damage, shortened telomeres, or oncogenic stress, both the p53 pathway and the NF-κB pathway are activated by ATM. p53 then “drives” cell senescence, whereas the NF-κB pathway promotes senescent cell clearance via STAT3 mediated production of over 100 genes called the “SASP,” aka the “senescent associated secretory phenotype”. The main cytokine secreted with the SASP is IL-6. IL-6 signaling degrades p53 and reduces the production of more senescent cells. Here is where NF-κB is our “best friend and our worst enemy”, combined into one transcription factor!
Here is an oversimplified diagram of this:
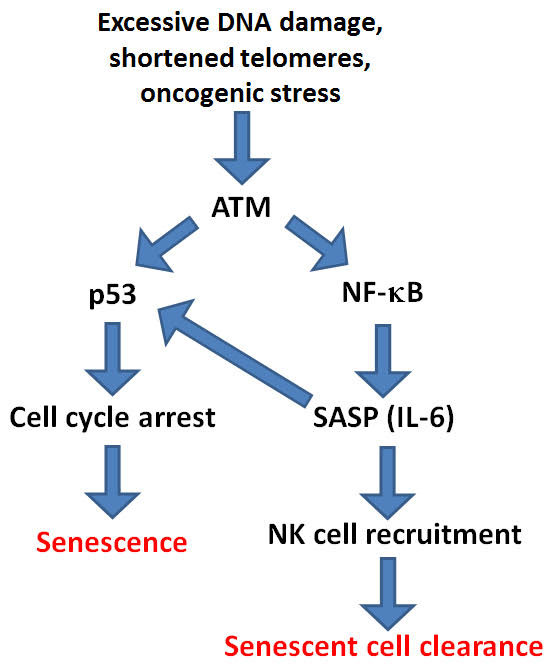
References: 2013 Modulation of immune responses by the tumor suppressor p53
From the 2011 publication Inflammation and p53 “Numerous observations indicate a strong link between chronic inflammation and cancer. This link is supported by substantial experimental evidence indicating mutual negative regulation of NF-κB , the major regulator of inflammation, and p53, the major tumor suppressor. This antagonistic relationship reflects the opposite principles of the physiological responses driven by these transcription factors, which act as sensors and mediators of intrinsic and extrinsic cell stresses, respectively. Constitutive activation of NF-κB , the underlying cause of chronic inflammation, is a common acquired characteristic of tumors. A variety of experimental methods have been used to demonstrate that constitutive activation of NF-κB reduces the tumor suppressor activity of p53, thereby creating permissive conditions for dominant oncogene-mediated transformation. Loss of p53 activity is also a characteristic of the majority of tumors and results in unleashed inflammatory responses due to loss of p53-mediated NF-κB suppression. On the other hand, in natural or pharmacological situations of enforced p53 activation, NF-κB activity, inflammation, and immune responses are reduced, resulting in different pathologies. It is likely that the chronic inflammation that is commonly acquired in various tissues of older mammals leads to general suppression of p53 function, which would explain the increased risk of cancer observed in aging animals and humans. Although the molecular mechanisms underlying reciprocal negative regulation of p53 and NF-κB remain to be deciphered, this phenomenon has important implications for pharmacological prevention of cancer and aging and for new approaches to control inflammation.”
SECTION C: CANCER-INFLAMMATION CROSS-LINKS AND HOW PLANT-BASED EXTRACTS MAY HELP WITH BOTH
By James P Watson
The situation described above, as is too-often the case with these blog entries, can on additional scrutiny get more and more complicated. In this Section I look particularly at what potentially can be done to control cancer via controlling inflammation using certain plant-based natural substances considering inflammasomes as well as p53, NF-κB and Nrf2.
1. p53 activates NF-κB – the cancer-inflammation link
In cells where there is DNA damage due to excessive mitochondrial ROS, UV light, X-ray/gamma-ray radiation, or oncogenic stress, p53 is activated and activates NF-kB. Sesquiterpine lactones like Arglabin can inhibit this activation of NF-κB . This is how constitutive activation of p53 results in constitutive activation of NF- B! Unfortunately, activating NF-κB actually promotes cancer cell survival! This is the “double edged sword”. For this reason, many cancer researchers have advocated the simultaneous targeting of p53 and NF-κB (I.e. Activating p53 and inhibiting NF-κB simultaneously). However, this strategy is also a “double-edged sword”.
Conclusion: activating p53 and inhibiting NF- B at the same time may be a good strategy for preventing or treating cancer.
Here is a diagram on this.
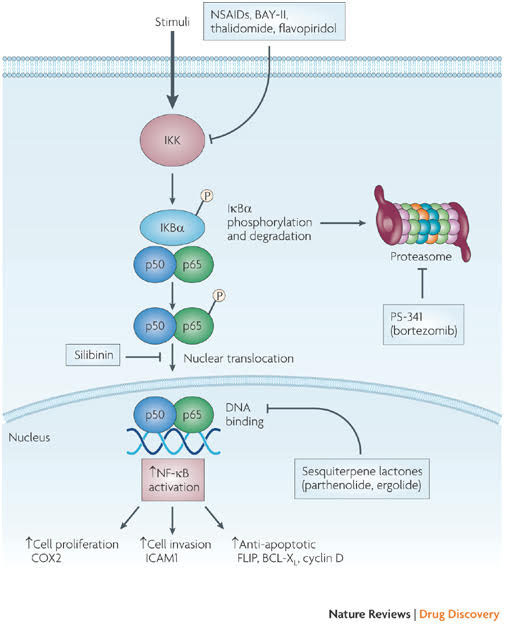
Image and legend source: Classes of compounds that simultaneously activate p53 and inhibit NF-κB “Nuclear factor- B (NF-κB) is activated by more than 200 different stimuli. These lead to the activation of a 900 kDa I B kinase (IKK) complex. The main substrate of IKK is IκB , which functions as an inhibitor of NF-κB transcriptional activity. Degradation of IκBa releases NF-κB (a dimer of two subunits; shown here are the p50 and p65 subunits, which are most common in cells), which is further covalently modified. Covalent modifications, such as phosphorylation, of NF-κB aid the recruitment of chromatin remodelling enzymes. Activation of NF-κB can result in several consequences and the most relevant ones include upregulation of genes that are involved in cell proliferation, cell invasion and cell death (anti-apoptotic genes). Some covalent modifications have been reported to recruit histone deacetylases and therefore turn off transcription. Inhibition of NF-κB can be achieved by inhibiting multiple steps along the pathway. Some of the nodal points for which inhibitors have been designed are indicated and representative examples of inhibitors for each category are shown in blue boxes. Several hundred inhibitors are known to target this pathway and many are in various stages of clinical trials6. COX2, cyclooxygenase 2; FLIP, also known as CFLAR; ICAM1, intracellular adhesion molecule 1; MEK, MAPK/ERK kinase; NSAIDs, non-steroidal anti-inflammatory drugs.”
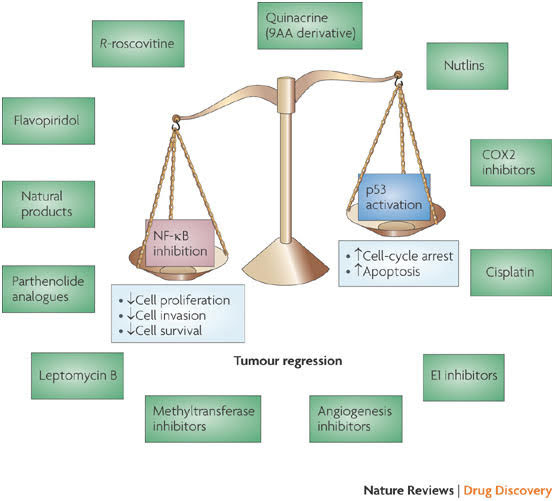
Image and legend source: Classes of compounds that simultaneously activate p53 and inhibit NF=κB “Several compounds that have been shown to activate p53 while inhibiting nuclear factor- B (NF-κB) are depicted here. Activating p53 promotes cell-cycle arrest and apoptosis, whereas inhibiting NF-κB decreases cell proliferation, cell invasion and cell survival, thereby promoting tumour regression. —”
Illustration explanation:
“The p53 and nuclear factor-κB (NF-κB) pathways play crucial roles in human cancer, in which inactivation of p53 and hyperactivation of NF-κB is a common occurrence. Activation of p53 and inhibition of NF-κB promotes apoptosis. Although drugs are being designed to selectively activate p53 or inhibit NF-κB, there is no concerted effort yet to deliberately make drugs that can simultaneously do both. Recent results suggest that a surprising selection of small molecules have this desirable dual activity. In this Review we describe the principles behind such dual activities, describe the current candidate molecules and suggest mechanisms and approaches to their further development.”
Reference: 2011 Inflammation and p53 – A Tale of Two Stresses “Numerous observations indicate a strong link between chronic inflammation and cancer. This link is supported by substantial experimental evidence indicating mutual negative regulation of NF-κB, the major regulator of inflammation, and p53, the major tumor suppressor. This antagonistic relationship reflects the opposite principles of the physiological responses driven by these transcription factors, which act as sensors and mediators of intrinsic and extrinsic cell stresses, respectively. Constitutive activation of NF-κB, the underlying cause of chronic inflammation, is a common acquired characteristic of tumors. A variety of experimental methods have been used to demonstrate that constitutive activation of NF-κB reduces the tumor suppressor activity of p53, thereby creating permissive conditions for dominant oncogene-mediated transformation. Loss of p53 activity is also a characteristic of the majority of tumors and results in unleashed inflammatory responses due to loss of p53-mediated NF-κB suppression. On the other hand, in natural or pharmacological situations of enforced p53 activation, NF-κB activity, inflammation, and immune responses are reduced, resulting in different pathologies. It is likely that the chronic inflammation that is commonly acquired in various tissues of older mammals leads to general suppression of p53 function, which would explain the increased risk of cancer observed in aging animals and humans. Although the molecular mechanisms underlying reciprocal negative regulation of p53 and NF-κB remain to be deciphered, this phenomenon has important implications for pharmacological prevention of cancer and aging and for new approaches to control inflammation.”
2. Parthenolides inhibit the Inflammasome directly and also inhibit NF-κB
This is a major reason why I am so excited about this class of natural compounds. There are many of these sesquiterpine that inhibit the NF-lab pathway and half a dozen that also inhibit the NLRP3 Inflammasome as well. Examples include the Parthenolide, derived from the Feverfew plant. Arglabin, derived from the Kazakh tree also is a sesquiterpine. Here are two illustrations of how these natural products (and Bay 11-7082) work on inhibiting NF-kB signaling and also work on inhibiting the NLRP3 Inflammasome.
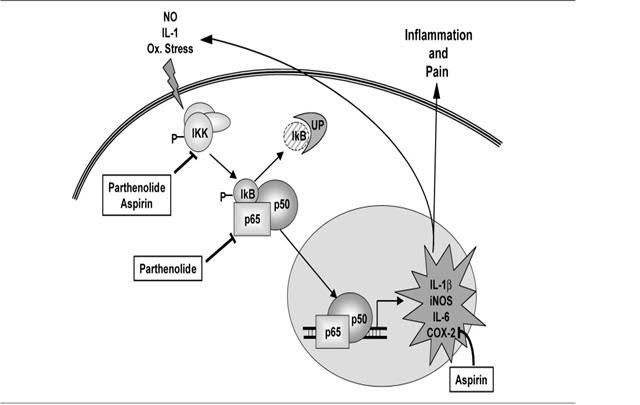
Image source: Tanacetum Parthenium (Feverfew) “
- “Parthenolide binds to and inhibits IκB kinase complex (IKK)β
- Parthenolide inhibits prostaglandine synthetase
- Parthenolide reduces human neutrophil oxidative burst activity”
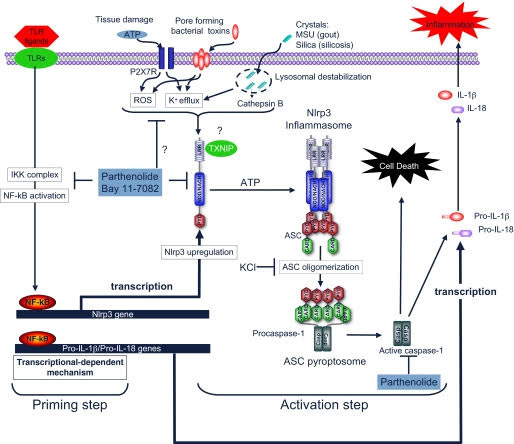
Reference and image source: 2010 Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome
Conclusions on inhibiting the Inflammasome directly and inhibitingNF-κB directly: It is now clear that inhibition if NF-κB is clearly a good target for preventing aging and reducing cancer risk. However most NF-κB inhibitors do not also directly inhibit Inflammasomes. Parthenolides are unique molecules in that they directly inhibit NF-κB activation by Toll-like receptors (TLRs), but also directly inhibit Inflammasomes by inhibiting Caspace activation and P2X7 receptor mediated potassium efflux from the cell. For this reason, Parthenolides and synthetic analogs may end up being very effective chemoorevention compounds for cancer and for aging.
3. Nrf2 and NF-κB crosstalk – why broccoli prevents cancer
Nrf2 is the key transcription factor that turns on most of the antioxidant enzyme genes (HO-1, SOD,1, NQ01), the genes involving glutathione synthesis (GCLM, GCLC, GCS, GSR), the genes involving NADPH synthesis (G6PD, malice enzyme), and free radical detoxification genes (GPX2, peroxiredoxin, and other enzymes that directly inactivate oxidants or electrophiles (GSTs, UGTs, etc.). Recent research has shown that there is considerable crosstalk between the Nrf2 pathway and several other critical stress sensing pathways, the most important ones being p53, Notch1, and NF-κB. As a consequence, most phytochemicals that activate Nrf2, such as the isothiocyanates found in broccoli, Brussel sprouts, cabbage, cauliflower, and watercress also suppress NF-κB. The opposite also appears to be true – most anti-inflammatory compounds that inhibit NF-κB also activate the Nrf2 pathway.
Image soure: 2010 Nrf2 and NF-κB and Their Concerted Modulation in Cancer Pathogenesis and Progression
References:
2014 The Nrf2 pathway in the progression of renal disease
2008 Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2- Keap1 signaling “This study demonstrates the involvement of Nrf2-Keap1 signaling through which EGCG enhances antioxidant activities and Phase II enzymes with subsequent restraint inflammation during bleomycin-induced pulmonary fibrosis.”
2007 Chalcone inhibits the activation of NF-κB and STAT3 in endothelial cells via endogenous electrophile
Conclusion: Nrf2 activators may have a synergistic effect on suppressing NF- kB.
4. p53 and Nrf2 crosstalk – p53 suppresses Nrf2-dependent transcription of antioxidant response genes
Just like there is crosstalk between p53 and NF-κB, there is also crosstalk between p53 and Nrf2. In 2006, researchers from Napoli, Italy showed that when DNA damage occurs and p53 is activated, the transcription of genes with antioxidant response elements (ARE) are suppressed. The ARE is the DNA binding sequence for the Nrf2 transcription factor. Thus they concluded that p53 hyperactivation results in the inhibition of Nrf2-dependent transcription of antioxidant genes such as NQ01, GST-a1, and x-CT.
Why did nature (Evolution) develop this crosstalk pathway that seems to be counter active to cell survival?
Well, the answer is obvious if we think of what the two major functions of p53 are. Most of the time we think of p53 is a pathway that turns off the cell cycle, but with extensive DNA damage, p53 actually triggers cell suicide (apoptosis), to prevent cancer from developing. One way that p53 does this (apoptosis) is by activating pro-apoptotic genes which increase mitochondrial-induced ROS leak out of the mitochondria. If the Nrf2 pathway allowed antioxidant genes to be upregulated in response to this ROS leak, these DNA damaged cells would become “ROS resistant” and the p53 “suicide program” would not work! We see this occurring in cancer cells that upregulate the Nrf2 pathway and thus become apoptosis resistant!
If this part of the Tale of Three Stress Sensors was that simple, we could just end the discussion now, but more research since 2006 has made the picture much more complicated. In 2012, researchers from Istanbul, Turkey, showed that p53 is both a positive and negative regulator of ROS production. Specifically, they showed that a tightly regulated feedback loop exists between ROS and FOXO genes, such as FOXO3a. Here ROS increases FOXO-mediated transcription if antioxidant genes such as SOD1, which makes cells ROS resistant.
Reference: 2012 Tumor suppressor genes and ROS: complex networks of interactions
That same year, a new review article from another research group in Rome and the U.K. supported this same paradoxical view of p53 and ROS.
Here they showed that when the cell is not under genotoxic stress, p53 is expressed at low levels because it is degraded by MDM2-mediated 26S proteasomal degradation as well as by NQ01-regulated ubiquitin-independent 20S proteasomal degradation. (Remember that the p53 protein is an intrinsically disordered protein, IDP, and that these proteins are regulated by their degradation rate, not their synthesis rate). At these low levels of p53 expression, three genes are induced by p53 that are actually antioxidant stress proteins (SESN1, SESN2, GPX, and GLS2). When the p53 gene is “knocked out” in cell studies, animal models, and in cancer, there is actually an increase in ROS due to the lack of expression of SESN1, SESN2, and GPX (due to p53 loss). Thus, p53 knock-out increases ROS, which increases DNA damage and further mutates genes. This is why tumors with p53 loss-of-function mutations have more rapid gene mutation rates.
Here is an illustration of this.
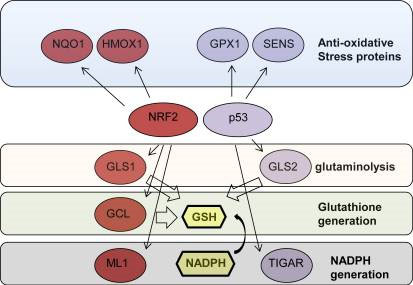
The story gets even more complicated, however.
Because p53 is such a complex molecule with so many binding partners and so many possible post translational modifications, other researchers tried to sort out the specific reasons for the pleiotropic effects of p53, based on lysine acetylation of specific residues on the p53 protein. They identified three p53 lysine sites that are often acetylated. By mutating the p53 gene, which replaced these three lysines with arginine amino acids, they were able to impair the ability of the mutant p53 protein from executing the cell cycle arrest, apoptosis, and cell senescence programs. To everyone’s surprise, these p53 mutants still retained their tumor suppressor function and these p53 mutant mice did not develop cancer! What they found was that these p53 mutants still could induce two genes that acted as tumor suppressors – TIGAR and GLS2.
TIGAR is a p53-induced glycolysis and apoptosis regulator gene. GLS2 is a gene that regulates energy metabolism and antioxidant genes, such as the GSH gene, which regulates glutathione levels within the cell.
References:
2010 Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function
2006 TIGAR, a p53-inducible regulator of glycolysis and apoptosis
2012 NRF2 and p53: Januses in cancer? “The transcription factor nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2 or NRF2, is a master regulator of the anti-oxidative stress response and positively controls the expression of a battery of anti-oxidative stress response proteins and enzymes implicated in detoxification and glutathione generation. Although its detoxifying activity is important in cancer prevention, it has recently been shown that cancer cells also exploit its protective functions to thrive and resist chemotherapy. NRF2 was also shown to the pentose phosphate pathway and glutaminolysis, which promotes purine synthesis for supporting rapid proliferation and glutathione for providing anti-oxidative stress protection. Evidence obtained from cancer patients and cell lines suggest that NRF2 is highly active in a variety of human cancers and is associated with aggressiveness. p53 is a tumor suppressor that also promotes an anti-oxidative stress metabolic program and glutaminolysis. H ere we will discuss the similarities between NRF2 and p53 and review evidence that p53 might be exploited by cancer cells to gain protection against oxidative stress, as is the case for NRF2. We discuss findings of co-regulation between these transcription factors and propose possible therapeutic strategies that can be used for treatment of cancers that harbor WT p53 and express high levels of NRF2.”
2006 p53 Suppresses the Nrf2-dependent Transcription of Antioxidant Response Genes* “Cells respond to the shift of intracellular environment toward pro-oxidant conditions by activating the transcription of numerous “antioxidant” genes. This response is based on the activation of the Nrf2 transcription factor, which transactivates the genes containing in their promoters the antioxidant response cis-elements (AREs). If the oxidative stress provokes DNA damage, a second response of the cell takes place, based on the activation of p53, which induces cell cycle arrest and/or apoptosis. Here we have explored the cross-talk between these two regulatory mechanisms. The results show that p53 counteracts the Nrf2-induced transcription of three ARE-containing promoters of the x-CT, NQO1, and GST-α1 genes. Endogenous transcripts of these antioxidant genes accumulate as a consequence of Nrf2 overexpression or exposure to electrophile diethylmaleate, but these effects are again blocked by p53 overexpression or endogenous p53 activation. Chromatin immunoprecipitation experiments support the hypothesis that this p53-dependent trans-repression is due to the direct interaction of p53 with the ARE-containing promoters. Considering that p53-induced apoptosis requires an accumulation of reactive oxygen species, this negative control on the Nrf2 transactivation appears to be aimed to prevent the generation of a strong anti-oxidant intracellular environment that could hinder the induction of apoptosis.”
Reference: 2012 NRF2 and p53: Januses in cancer?
Conclusions on #4: To prevent cancer from occurring, p53 will trigger cell suicide when there is too much DNA damage (aka apoptosis). This p53 suicide program includes Mitochondrial-mediated ROS production. To prevent the Nrf2 pathway from counteracting this suicide program, p53 suppresses antioxidant gene expression by preventing the Nrf2 pathway from activating antioxidant gene expression. This occurs not only with cancer, but also with aging, where p53 triggers cellular senescence. Thus this p53-mediated suppression of Nrf2 pathway is a major unsolved problem in aging.
5. Crosstalk between Nrf2 and oncogenes
Unfortunately, cancer cells have learned to exploit the Nrf2 pathway to help them survive. Whereas this can occur via p53, it can also occur via several other mechanisms. For instance, the oncogene called K-Ras can increase the expression of Nrf2 and thereby increase antioxidant enzyme production. As a consequence, these K-Ras cancers become very hard to treat with either genotoxic chemotherapy or radiation. It is this cancer exploitation of the Nrf2 pathway which has made anti-aging researchers reluctant to give Nrf2 the classification as a “longevity gene.”
Reference: 2011 Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis
Conclusion of #5: The Nrf2 pathway can be exploited by cancers to promote ROS resistance, which makes these tumors resistant to apoptosis by chemotherapy and radiation therapy. A good example of such cancers are those that over express the K-Ras oncogene, which upregulated the Nrf2 pathway. K-Ras mutations occur in >90% of pancreatic cancers. This may be why they rarely are cured with chemotherapy. Thus the Nrf2 pathway can hurt us in pancreatic cancer and other cancers which upregulate the Nrf2 pathway. This is why the Nrf2 pathway is a double-edged sword, which can hurt us or help us with cancer and aging.
6. Sesquiterpine Lactones inhibit tumor suppressor gene silencing – DNMT1 induced DNA hypermethylation of CpG Islands in the promoters of tumor suppressor genes can be treated or prevented with Arglabin
Much has been said recently about “DNA methylation clocks” such as the Horvath clock, which have been shown to correlate independently with age (R = 0.97) and also accurately predict lifespan. (See our recent blog entry Aging, health and disease – view from the DNA Methylome.) These computer-algorithm-derived “CpG clocks) contain both genome-wide residues that undergo age-dependent demethylation (global phenomena) and locus-specific residues that undergo age-dependent hypermethyation. The latter are usually found within CpG islands of promoters or enhancers. Recently, a group showed that parthenolides such as the molecules found in feverfew and Arglabin can inhibit the DNA hypermethylation of tumor suppressor promoters by the DNA methyltransferase, DNMT1. Thus sesquiterpine Smaug not only inhibit inflammation, they may also prevent cancer by preventing epigenetic tumor suppressor gene silencing. Whether these sesquiterpine will have the same effect on the “DNA methylation clock” for age-related CpG residues is still unknown. Here is some data and an illustration:
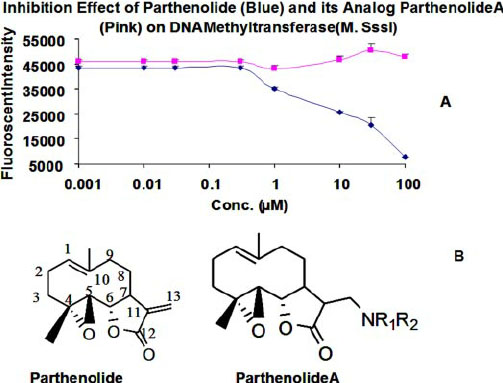
Reference: 2010 Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome “Activation of the inflammasome generates the pro-inflammatory cytokines interleukin-1β and -18, which are important mediators of inflammation. Abnormal activation of the inflammasome leads to many inflammatory diseases, including gout, silicosis, neurodegeneration, and genetically inherited periodic fever syndromes. Therefore, identification of small molecule inhibitors that target the inflammasome is an important step toward developing effective therapeutics for the treatment of inflammation. Here, we show that the herbal NF-κB inhibitory compound parthenolide inhibits the activity of multiple inflammasomes in macrophages by directly inhibiting the protease activity of caspase-1. Additional investigations of other NF-κB inhibitors revealed that the synthetic IκB kinase-β inhibitor Bay 11-7082 and structurally related vinyl sulfone compounds selectively inhibit NLRP3 inflammasome activity in macrophages independent of their inhibitory effect on NF-κB activity. In vitro assays of the effect of parthenolide and Bay 11-7082 on the ATPase activity of NLRP3 demonstrated that both compounds inhibit the ATPase activity of NLRP3, suggesting that the inhibitory effect of these compounds on inflammasome activity could be mediated in part through their effect on the ATPase activity of NLRP3. Our results thus elucidate the molecular mechanism for the therapeutic anti-inflammatory activity of parthenolide and identify vinyl sulfones as a new class of potential therapeutics that target the NLRP3 inflammasome.”
Reference: 2009 Regulation_of_DNA_Methylation_by_a_Sesquiterpene_Lactone_Parthenolide


Pingback: ON AGING - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: Inflammation Part 3: resolving inflammation - resolvins, protectins, maresins and lipoxins - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: Inflammation Part 4 - PCSK9 inhibition - Also Part 1 of interventions that reduce all cause mortality (ACM) - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: INFLAMMATION PART 6: THE SCIENCE BEHIND THE 4 HERB SYNERGY DIETARY SUPPLEMENT - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: UPDATE ON LONGEVITY INTERVENTIONS – MAINLY PERSONAL - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: Inflammation Part 5: Inflammasomes – science of and disease implications - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™
Pingback: Inflammation Part 7: Neurohormesis, neuroinflammatory diseases, and their treatment by mushroom substances (Section 1) - AGINGSCIENCES™ - Anti-Aging Firewalls™AGINGSCIENCES™ – Anti-Aging Firewalls™